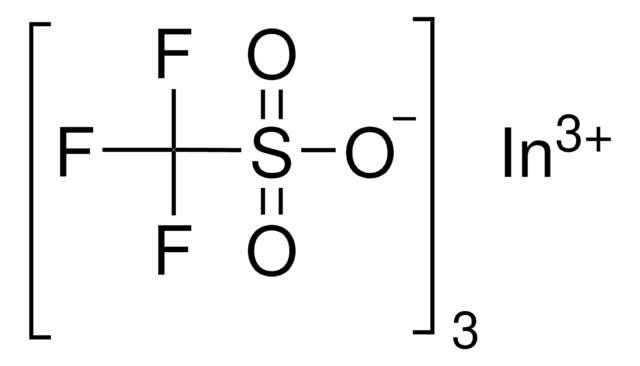
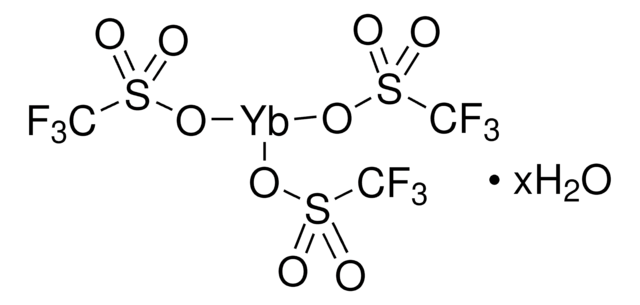
425176
Gadolinium(III) trifluoromethanesulfonate
98%
Synonym(s):
Tris(trifluoromethanesulfonato)gadolinium, Gadolinium(III) triflate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CF3SO3)3Gd
CAS Number:
Molecular Weight:
604.46
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
reaction suitability
core: gadolinium
reagent type: catalyst
SMILES string
[Gd+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Gd/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
DYOBTPTUHDTANY-UHFFFAOYSA-K
Application
A water-tolerant Lewis acid used in the Aldol reaction of silyl enol ethers with aldehydes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yamanaka et al.
Organic letters, 2(2), 159-161 (2000-05-18)
[reaction: see text] Ytterbium trifluoromethanesulfonate [Yb(OTf)3] catalyzed the imino ene reaction of N-tosyl aldimine with alpha-methylstyrene to give a homoallylamine in moderate yield. Furthermore, addition of a catalytic amount of chlorotrimethylsilane (TMSCI) dramatically enhanced the imino ene reaction.
Tomoaki Hamada et al.
Journal of the American Chemical Society, 125(10), 2989-2996 (2003-03-06)
Catalytic asymmetric aldol reactions in aqueous media have been developed using Pr(OTf)(3) and chiral bis-pyridino-18-crown-6 1. In the asymmetric aldol reaction using rare earth metal triflates (RE(OTf)(3)) and 1, slight changes in the ionic diameters of the metal cations greatly
David A Evans et al.
Journal of the American Chemical Society, 125(34), 10162-10163 (2003-08-21)
A highly enantioselective, quinone Diels-Alder reaction catalyzed by chiral samarium and gadolinium pyridyl-bis(oxazoline) (pybox) complexes has been developed. The reaction scope has been extended to include three quinones and five dienes, all of which exclusively provide the expected endo product
Kobayashi, S.; Nagayama, S.
Journal of the American Chemical Society, 119, 10049-10049 (1997)
The Journal of Organic Chemistry, 59, 3590-3590 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service