376469
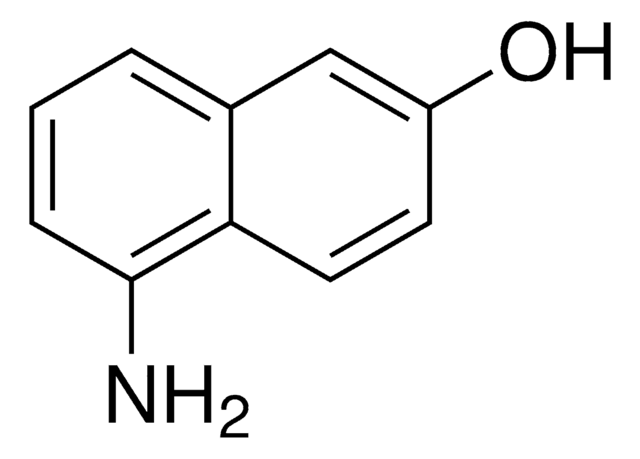
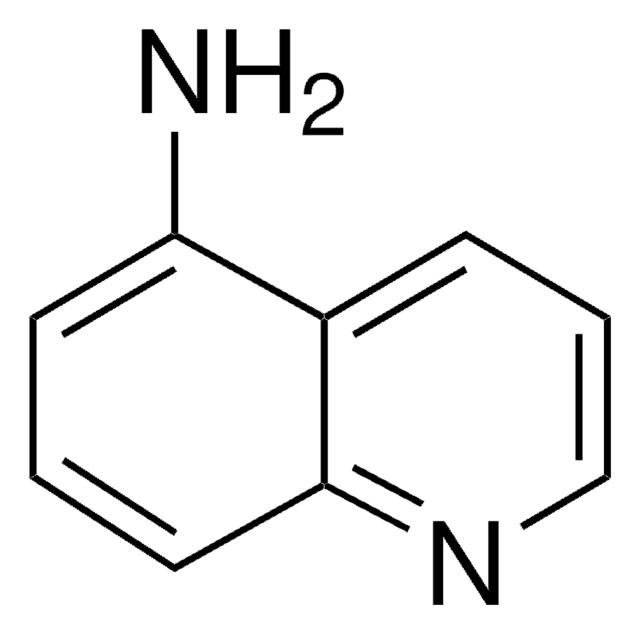
5-Amino-1-naphthol
95%
Synonym(s):
1-Amino-5-hydroxynaphthalene, 1-Amino-5-naphthol, 5-Amino-α-naphthol, 5-Amino-1-naphthalenol, 5-Hydroxy-1-naphthalenamine, 5-Hydroxy-1-naphthylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC10H6OH
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
190 °C (dec.) (lit.)
SMILES string
Nc1cccc2c(O)cccc12
InChI
1S/C10H9NO/c11-9-5-1-4-8-7(9)3-2-6-10(8)12/h1-6,12H,11H2
InChI key
ZBIBQNVRTVLOHQ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ewa Rozycka-Sokolowska et al.
Acta crystallographica. Section C, Crystal structure communications, 65(Pt 11), o565-o568 (2009-11-07)
The crystal structure of the title compound, C(10)H(9)NO, (I), contains intermolecular O-H...N and N-H...O hydrogen bonds which together form sheets parallel to the (001) plane containing rings with an unusual R(4)(4)(18) motif. These rings are additionally stabilized by an intermolecular
Study of a heteropolyanion-doped poly (5-amino-1-naphthol) film electrode and its catalytic activity.
Pham M-C, et al.
Electrochimica Acta, 42(3), 439-447 (1997)
Dharam Paul Jindal et al.
Arzneimittel-Forschung, 52(9), 654-663 (2002-10-31)
The object of this study was to investigate the beta-adrenergic receptor binding affinity of 4-acylaminophenoxypropanolamine (10-15) and 5-acylaminonaphthyloxypropanolamine (21-24) derivatives, which were prepared from 4-aminophenol (5) and 5-amino-1-naphthol (16), respectively. The in vitro beta 1- and beta 2-adrenergic receptor binding
P M Enriquez et al.
Toxicology, 29(4), 337-343 (1984-02-01)
The metabolic fate of [14C]5-amino-1-naphthol (5A1N) was investigated in Sprague-Dawley rats. [14C]5A1N was administered by gastric intubation to male rats at doses 1, 37 and 135 mg/kg body weight. In a separate experiment the rats were also dosed with 150
Electrochemical synthesis and study of poly (5-amino 1-naphthol) film in aqueous and organic media.
Pham M-C, et al.
Synthetic Metals, 63(1), 7-15 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service