365386
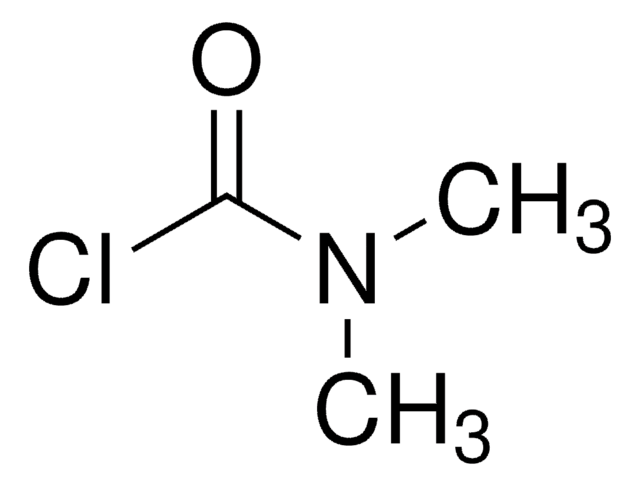
N,N-Diisopropylcarbamoyl chloride
98%
Synonym(s):
Diisopropylcarbamic chloride, Diisopropylcarbamyl chloride, N,N-Bis(1-methylethyl)carbamic chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
[(CH3)2CH]2NCOCl
CAS Number:
Molecular Weight:
163.65
Beilstein:
1754834
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
90-93 °C/15 mmHg (lit.)
mp
57-59 °C (lit.)
functional group
amine
chloro
SMILES string
CC(C)N(C(C)C)C(Cl)=O
InChI
1S/C7H14ClNO/c1-5(2)9(6(3)4)7(8)10/h5-6H,1-4H3
InChI key
RSAFAYLZKCYUQW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N-Diisopropylcarbamoyl chloride may be used in the preparation of:
- 1-aryl-1-alkenyl N,N-diisopropylcarbamates
- 1,3-diphenylallyl carbamate
- (Z)-1,3-diphenyl-1-propenyl N,N-diisopropylcarbamate
- (R)-1-(2-methoxy-4-methylphenyl)ethyl diisopropyl-carbamate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asymmetric ?-Deprotonation and Substitution Reactions of (Z)-1, 3-Diphenyl-1-propenyl N, N-Diisopropylcarbamate.
Reuber J, et al.
European Journal of Organic Chemistry, 14, 3017-3025 (2005)
Stereoselective Intermolecular Carbolithiation of Open-Chain and Cyclic 1-Aryl-1-alkenyl N,N-Diisopropylcarbamates Coupled with Electrophilic Substitution. Observation of p-Carboxylation in a Benzyllithium Derivative.
Peters JG, et al.
Synthesis, 03, 0381-0292 (2002)
The total synthesis of (-)-aplysin via a lithiation-borylation-propenylation sequence.
Fletcher CJ, et al.
Tetrahedron, 68(23), 7598-7604 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service