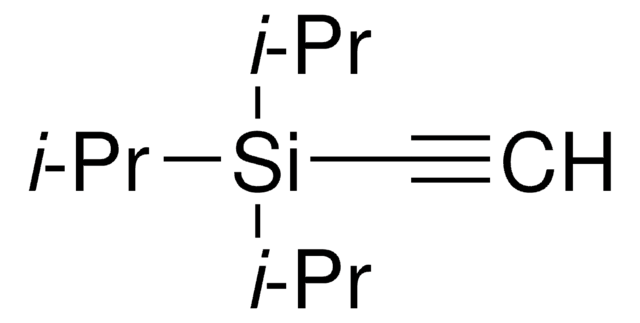360058
(Triphenylsilyl)acetylene
98%
Synonym(s):
1,1′,1′′-(Ethynylsilylidyne)tris[benzene], Ethynyltriphenylsilane, Triphenylsilylacetylene, Triphenylsilylethyne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
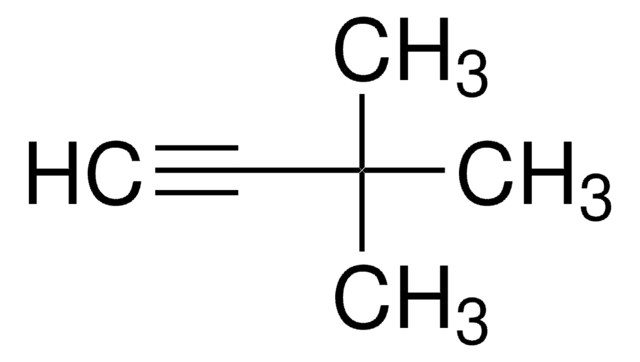
(C6H5)3SiC≡CH
CAS Number:
Molecular Weight:
284.43
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
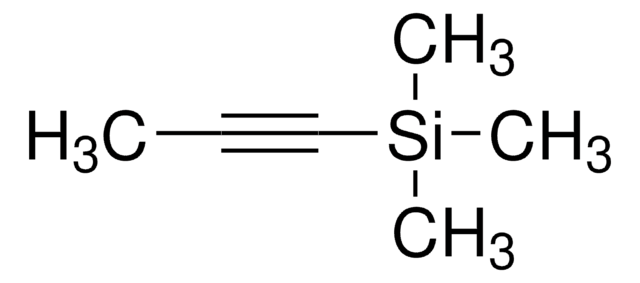
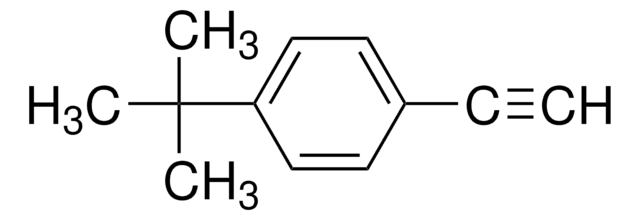
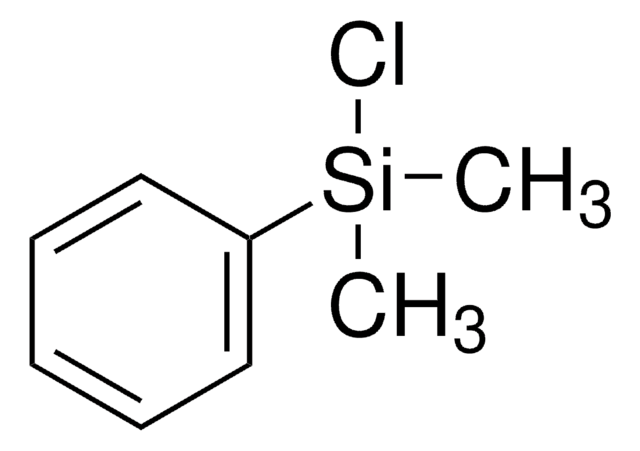
Recommended Products
Quality Level
Assay
98%
mp
48-50 °C (lit.)
SMILES string
C#C[Si](c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C20H16Si/c1-2-21(18-12-6-3-7-13-18,19-14-8-4-9-15-19)20-16-10-5-11-17-20/h1,3-17H
InChI key
WADKYPSVXRWORK-UHFFFAOYSA-N
General description
(Triphenylsilyl)acetylene is a terminal alkyne. Rhodium-catalyzed asymmetric addition of (triphenylsilyl)acetylene to diphenylphosphinylallene is reported.
Application
(Triphenylsilyl)acetylene may be used in the synthesis of:
- selenothioic acid S-alkyl esters
- methyl 2-(di{ 3, 5-bis[(triphenylsilyl)ethynyl]phenyl}-phosphino)benzoate
- 2-(di{3,5-bis[(triphenylsilyl)ethynyl]phenyl}-phosphino)benzoic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Takahiro Nishimura et al.
Chemistry, an Asian journal, 3(8-9), 1505-1510 (2008-05-09)
The presence of an acid was found to be essential in the rhodium-catalyzed asymmetric addition of terminal alkynes to diarylphosphinylallenes giving exo-enynes in high yields with high regio- and enantioselectivity. The stereochemical outcome is determined at the protonolysis of the
Selenothioic acid S-esters: Synthesis, characterization, and trend for stability.
Murai T, et al.
Journal of the American Chemical Society, 119(37), 8592-8597 (1997)
Crafting chiral space. The synthesis of C~ 2-symmetric diphosphine ligands for an outer-sphere catalytic reaction.
Trost BM and Marschner C.
Bulletin de la Societe Chimique De France, 134, 263-274 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service