331910
2-Azabicyclo[2.2.1]hept-5-en-3-one
98%
Synonym(s):
(±)-2-Azabicyclo[2.2.1]hept-5-en-3-one, 4-Amino-2-cyclopentene-1-carboxylic acid lactam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
Beilstein:
508342
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
102-106 °C/0.25 mmHg (lit.)
mp
54-58 °C (lit.)
SMILES string
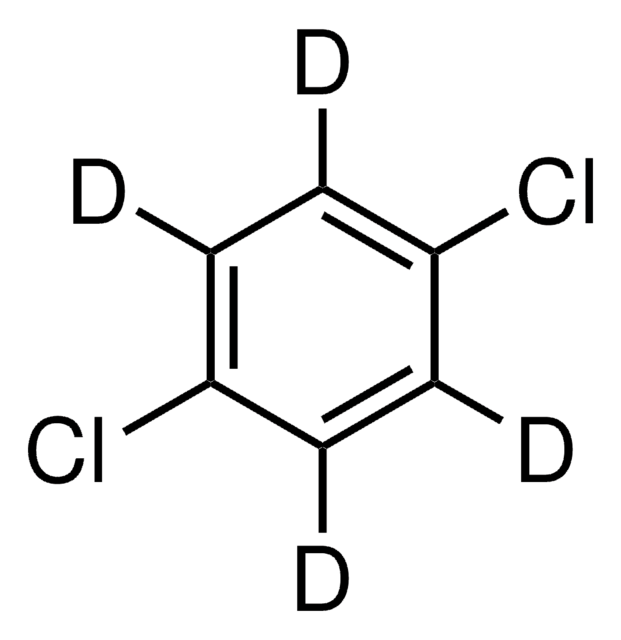
O=C1N[C@H]2C[C@@H]1C=C2
InChI
1S/C6H7NO/c8-6-4-1-2-5(3-4)7-6/h1-2,4-5H,3H2,(H,7,8)/t4-,5+/m0/s1
InChI key
DDUFYKNOXPZZIW-CRCLSJGQSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Azabicyclo[2.2.1]hept-5-en-3-one is also referred as vince lactam. It is a versatile intermediate in the synthesis of carbocyclic nucleosides. 2-Azabicyclo[2.2.1]hept-5-en-3-one and its monohydrated complex was investigated in a supersonic jet by Fourier transform microwave spectroscopy.
Application
2-Azabicyclo[2.2.1]hept-5-en-3-one was used in:
- preparation of (3aS,4R,6R,6aR)-6-((methoxy-methoxy)methyl)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-amine, a precursor of analog of bredinin
- synthesis of therapeutic drugs
- chemoenzymatic synthesis of (−)-carbovir
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chemoenzymatic synthesis of (-)-carbovir utilizing a whole cell catalysed resolution of 2-azabicyclo [2.2. 1] hept-5-en-3-one.
Steven, J. C.
Journal of the Chemical Society. Chemical Communications, 16, 1120-1121 (1990)
Patricia Écija et al.
The journal of physical chemistry. A, 116(41), 10099-10106 (2012-09-19)
2-Azabicyclo[2.2.1]hept-5-en-3-one (ABH or Vince lactam) and its monohydrated complex (ABH···H(2)O) have been observed in a supersonic jet by Fourier transform microwave spectroscopy. ABH is broadly used in the synthesis of therapeutic drugs, whereas the ABH···H(2)O system offers a simple model
Vasu Nair et al.
Molecules (Basel, Switzerland), 18(9), 11576-11585 (2013-09-21)
The natural nucleoside antibiotic, bredinin, exhibits antiviral and other biological activities. While various nucleosides related to bredinin have been synthesized, its carbocyclic analog has remained unknown. Synthesis of this heretofore unknown analog of bredinin is described. The key precursor, (3aS,4R,6R,6aR)-6-((methoxy-methoxy)methyl)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-amine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(1R)-(−)-2-Azabicyclo[2.2.1]hept-5-en-3-one ≥98%](/deepweb/assets/sigmaaldrich/product/structures/143/106/ed651024-4d6b-4603-9933-8a8f6e9f4dab/640/ed651024-4d6b-4603-9933-8a8f6e9f4dab.png)
![(1S)-(+)-2-Azabicyclo[2.2.1]hept-5-en-3-one ≥98%](/deepweb/assets/sigmaaldrich/product/structures/230/141/310ba46c-6b75-4ed3-9134-10ae806b9cc0/640/310ba46c-6b75-4ed3-9134-10ae806b9cc0.png)