307270
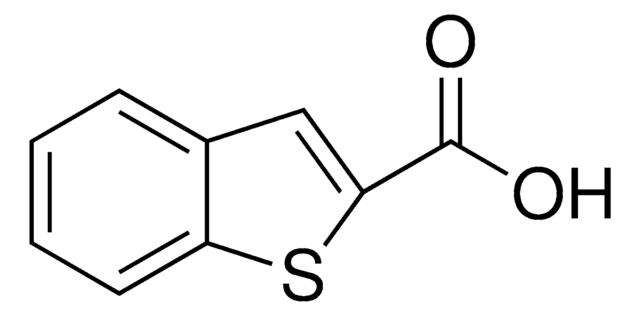
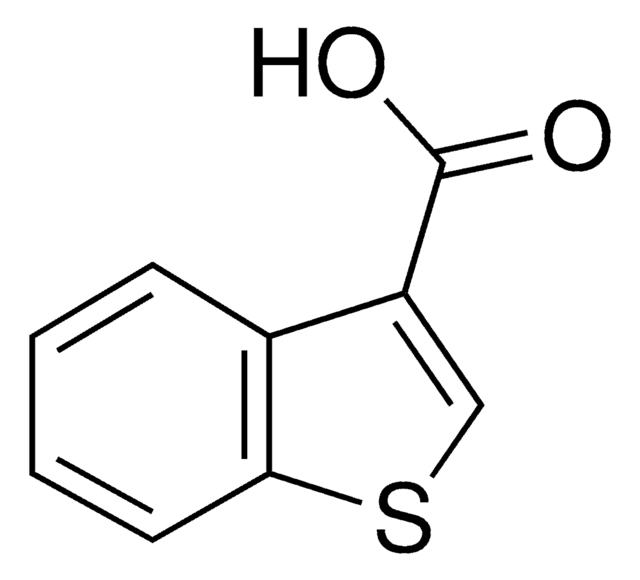
Benzofuran-2-carboxylic acid
99%
Synonym(s):
Coumarilic acid, Coumarone-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6O3
CAS Number:
Molecular Weight:
162.14
Beilstein:
124204
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
193-196 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cc2ccccc2o1
InChI
1S/C9H6O3/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5H,(H,10,11)
InChI key
OFFSPAZVIVZPHU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Benzofuran-2-carboxylic acid was used in the synthesis of the oxymethyl-modified coumarinic acid-based cyclic DADLE (H-Tyr-D-Ala-Gly-Phe-D-Leu-OH) prodrug.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yibin Xiang et al.
Bioorganic & medicinal chemistry letters, 21(10), 3050-3056 (2011-04-22)
Novel benzofuran-2-carboxylic acids, exemplified by 29, 38 and 39, have been discovered as potent Pim-1 inhibitors using fragment based screening followed by X-ray structure guided medicinal chemistry optimization. The compounds demonstrate potent inhibition against Pim-1 and Pim-2 in enzyme assays.
H Ouyang et al.
The journal of peptide research : official journal of the American Peptide Society, 59(4), 183-195 (2002-04-26)
The coumarinic acid-based cyclic DADLE (H-Tyr-D-Ala-Gly-Phe-D-Leu-OH) prodrug 1a exhibited more favorable physicochemical properties than did DADLE for permeation across the intestinal mucosa. However, prodrug 1a, whose bioconversion to DADLE was slow, was subject to extensive biliary clearance when administered to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service