275034
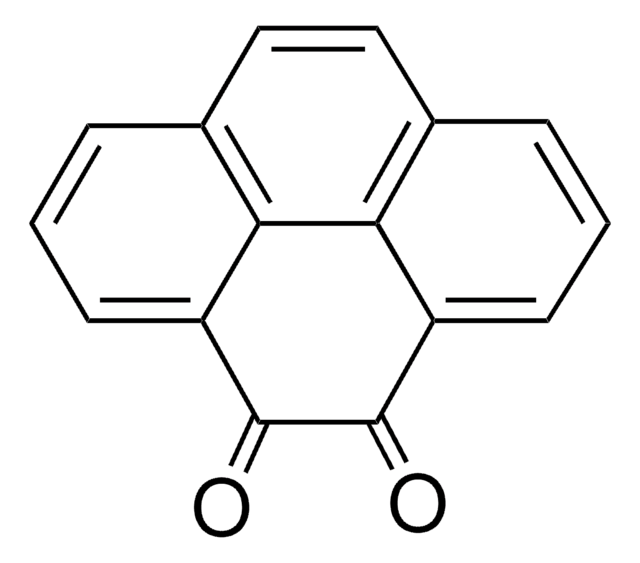
9,10-Phenanthrenequinone
95%
Synonym(s):
9,10-Phenanthrenedione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H8O2
CAS Number:
Molecular Weight:
208.21
Beilstein:
608838
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
95%
form
powder
mp
209-212 °C (lit.)
SMILES string
O=C1C(=O)c2ccccc2-c3ccccc13
InChI
1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H
InChI key
YYVYAPXYZVYDHN-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770) , PTPRC(5788)
Looking for similar products? Visit Product Comparison Guide
General description
The quinones of polycyclic aromatic hydrocarbons are present in abundance in all burnt organic material. On being used to passivate silicon surfaces, it reacts with the dangling bonds on the surface via a heteroatomic Diels-Alder reaction. On account of the Π-electron conjugation, the semi conducting nature of the silicon is unaffected.
Application
9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
473.0 °F
Flash Point(C)
245 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P L Chesis et al.
Proceedings of the National Academy of Sciences of the United States of America, 81(6), 1696-1700 (1984-03-01)
The mutagenicity of various quinones, a class of compounds widely distributed in nature, is demonstrated in the Salmonella TA104 tester strain. The metabolic pathways by which four quinones, menadione, benzo[a]pyrene 3,6-quinone, 9,10-phenanthrenequinone, and danthron, caused mutagenicity in this test system
Electronic structure and band alignment of 9, 10-phenanthrenequinone passivated silicon surfaces
Avasthi, Sushobhan, et al.
Surface Science, 605(13), 1308-1312 (2011)
Petr Milko et al.
Inorganic chemistry, 48(24), 11734-11742 (2009-11-26)
With the use of the model complexes [(PQ)FeCl(CH(3)O)](+), [(phen)FeCl(CH(3)O)](+), and [(PQ)(phen)FeCl(CH(3)O)](+), where PQ is 9,10-phenanthraquinone and phen is 1,10-phenanthroline, the reactivity of phenanthraquinone in complexes with iron(III) is investigated. It is shown that 9,10-phenanthraquinone takes part in redox processes occurring
Naoya Kishikawa et al.
Talanta, 85(1), 809-812 (2011-06-08)
9,10-Phenanthrenequinone (PQ) is harmful environmental pollutant that is detected in airborne particulates. The measurement of PQ in the air should be necessary to evaluate the potential adverse effects of PQ on human health. We have recently developed a determination method
Michael C Byrns et al.
Biochemical pharmacology, 75(2), 484-493 (2007-10-24)
Aldo-keto reductase (AKR) 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase) regulates ligand access to steroid hormone and prostaglandin receptors and may stimulate proliferation of prostate and breast cancer cells. NSAIDs are known inhibitors of AKR1C enzymes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service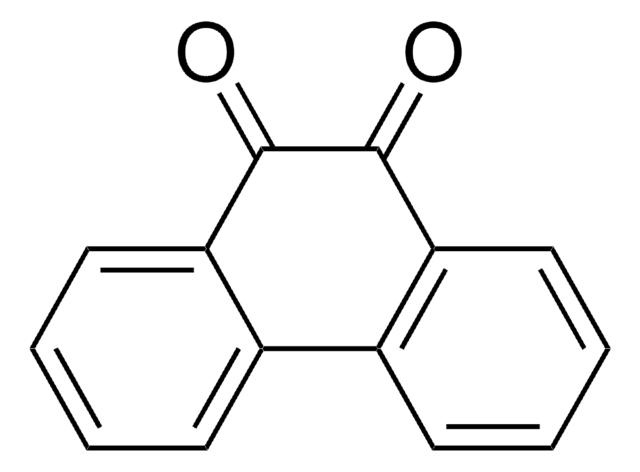





![6H-Benzo[cd]pyren-6-one BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/121/467/11adf097-4f11-4b40-a73f-910f36624e9c/640/11adf097-4f11-4b40-a73f-910f36624e9c.png)