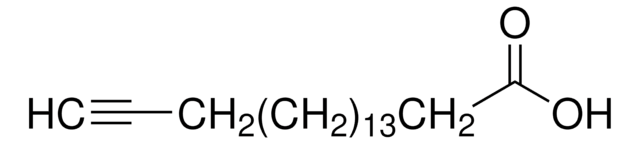232211
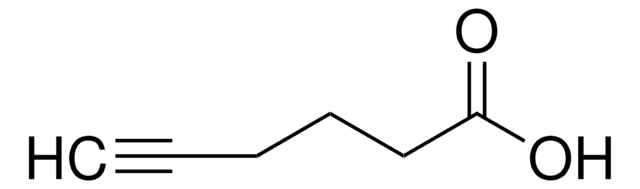
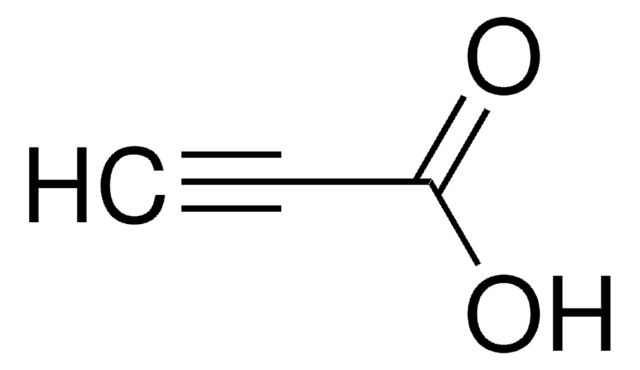
4-Pentynoic acid
95%
Synonym(s):
Propargylacetic acid
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI key
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as building block for the synthesis of library of eight sequence-defined model oligomers
- in one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives
- in the synthesis of various allenenols lactones [5(E)-(2-allenylidene)-tetrahydro-2-furanones]
- in the synthesis of a cyctotoxic macrolide by ring-closing metathesis of a bis acetylene
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
“Click” chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 232211-1G | 4061838783394 |
| 232211-250MG | |
| 232211-5G | 4061838783400 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service