All Photos(2)
About This Item
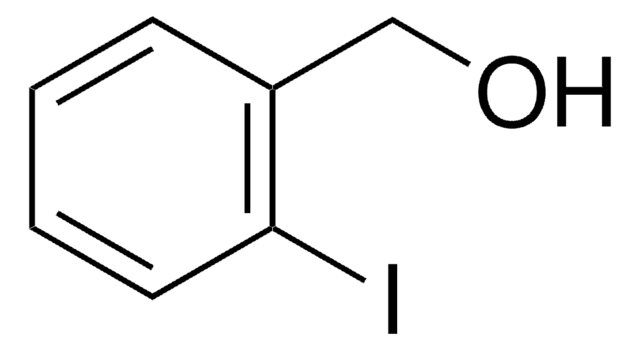
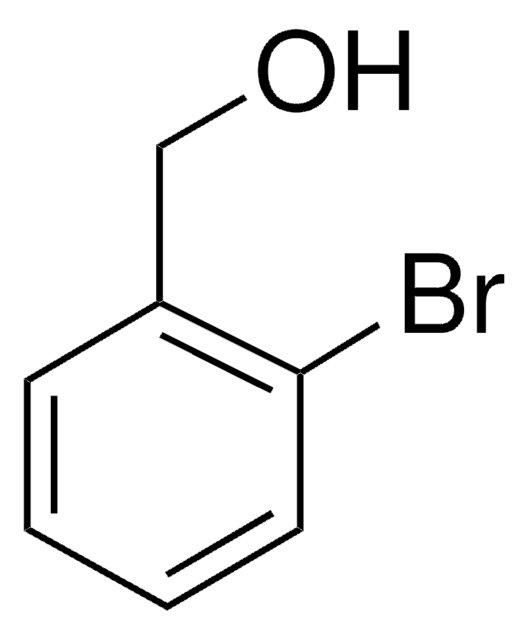
Linear Formula:
IC6H4CH2OH
CAS Number:
Molecular Weight:
234.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.636 (lit.)
bp
252 °C/711 mmHg (lit.)
density
1.842 g/mL at 25 °C (lit.)
functional group
hydroxyl
iodo
SMILES string
OCc1cccc(I)c1
InChI
1S/C7H7IO/c8-7-3-1-2-6(4-7)5-9/h1-4,9H,5H2
InChI key
QGCCNWSXJHGUNL-UHFFFAOYSA-N
General description
3-Iodobenzyl alcohol undergoes cross-coupling reaction with zinc reagent to yield 3R-tert-butoxycarbonylamino-4-(3-hydroxymethylphenyl)butanoic acid benzyl ester.
Application
3-Iodobenzyl alcohol was used in the preparation of:
- 6-(3-iodo-benzyloxy)-9H-purin-2-ylamine
- 3-(1-methylethenyl)benzenemethanol
- 3-ethenylbenzenemethanol
- dendritic iron(II) porphyrins
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Vaidyanathan et al.
Bioconjugate chemistry, 11(6), 868-875 (2000-11-23)
Two radiolabeled analogues of 6-benzyloxy-9H-purin-2-ylamine (O(6)-benzylguanine; BG) potentially useful in the in vivo mapping of O(6)-alkylguanine-DNA alkyltransferase (AGT) were synthesized. Fluorine-18 labeling of the known 6-(4-fluoro-benzyloxy)-9H-purin-2-ylamine (FBG; 6) was accomplished by the condensation of 4-[(18)F]fluorobenzyl alcohol with 2-aminopurin-6-yltrimethylammonium chloride (4)
Richard F W Jackson et al.
Organic & biomolecular chemistry, 2(1), 110-113 (2004-01-23)
Palladium-catalysed reaction of unprotected 2-, 3-, and 4-iodophenols with a range of amino acid derived organozinc reagents (not used in excess) gives the expected products in good to excellent yield, demonstrating that carbon-zinc bonds are not protonated by acidic phenols
1-Methyl-1-vinyl-and 1-Methyl-1-(prop-2-enyl) silacyclobutane: Reagents for Palladium-Catalyzed Cross-Coupling Reactions of Aryl Halides.
Denmark SE and Wang Z.
Synthesis, 2000(7), 999-1003 (2000)
Beatrice Felber et al.
Organic & biomolecular chemistry, 1(7), 1090-1093 (2003-08-21)
We report the synthesis of iron(II) porphyrins functionalised with first- and second-generation dendrons as mimics of haemoglobin. The porphyrin core bears an ethynyl linker pointing towards the centre of the molecule, in an ideal position for the introduction of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service