All Photos(2)
About This Item
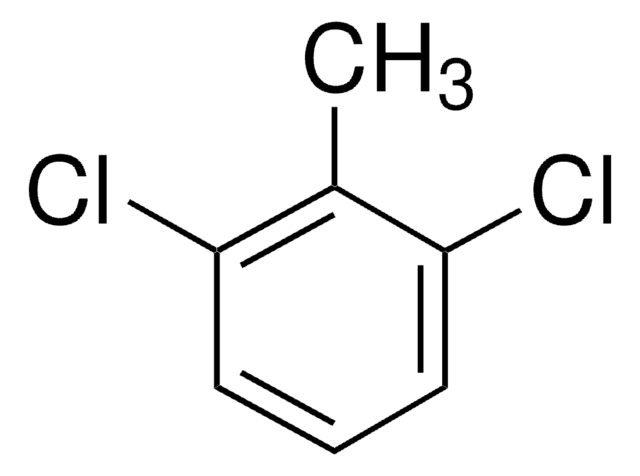
Linear Formula:
CH3C6H3Cl2
CAS Number:
Molecular Weight:
161.03
Beilstein:
1931687
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.547 (lit.)
bp
200.5 °C/741 mmHg (lit.)
density
1.251 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(Cl)c(Cl)c1
InChI
1S/C7H6Cl2/c1-5-2-3-6(8)7(9)4-5/h2-4H,1H3
InChI key
WYUIWKFIFOJVKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3,4-Dichlorotoluene is added as growth supplement in culture medium of Ralstonia sp. strain PS12.
Application
3,4-Dichlorotoluene was used in determination of enthalpy of formation for molecular complexes of I2 with chloromethylbenzene molecules in CCl4.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
185.0 °F
Flash Point(C)
85 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thermochemistry of molecular complexes. 4. Molecular complexes of I2 with chloromethylbenzenes.
Greaux JB, et al.
J. Incl. Phenom. Mol. Recog. Chem., 13(3), 245-248 (1992)
Katrin Pollmann et al.
Journal of bacteriology, 184(19), 5261-5274 (2002-09-10)
Ralstonia sp. strain PS12 is able to use 2,4-, 2,5-, and 3,4-dichlorotoluene as growth substrates. Dichloromethylcatechols are central intermediates that are formed by TecA tetrachlorobenzene dioxygenase-mediated activation at two adjacent unsubstituted carbon atoms followed by TecB chlorobenzene dihydrodiol dehydrogenase-catalyzed rearomatization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service