156671
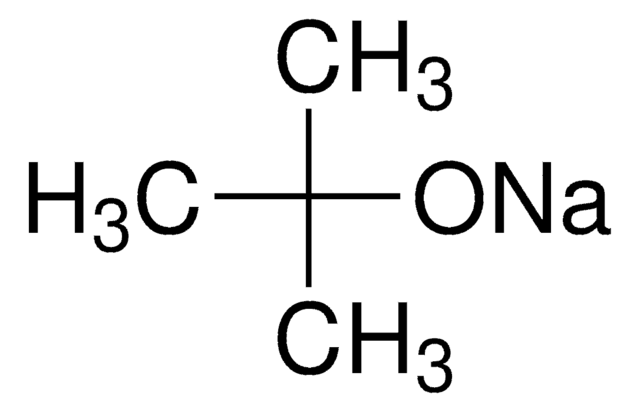
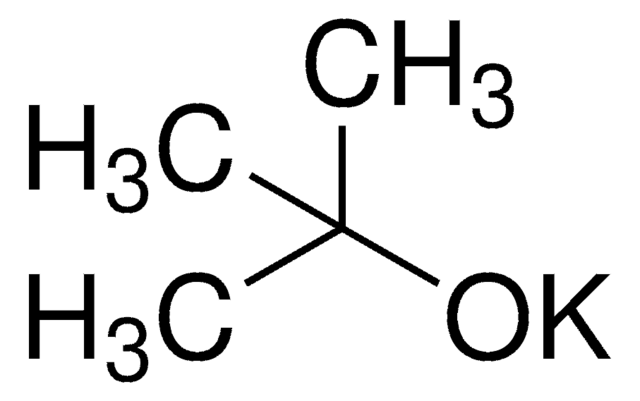
Potassium tert-butoxide
reagent grade, ≥98%
Synonym(s):
Potassium tert-butylate, Potassium t-butoxide
About This Item
Recommended Products
grade
reagent grade
Quality Level
vapor pressure
1 mmHg ( 220 °C)
Assay
≥98%
form
solid
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
256-258 °C (dec.) (lit.)
greener alternative category
SMILES string
[K+].CC(C)(C)[O-]
InChI
1S/C4H9O.K/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
LPNYRYFBWFDTMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It can also be used:
- To synthesize aliphatic and aromatic amides from corresponding esters and amines.
- As a base in the intramolecular cyclization of aryl ethers, amines, and amides.
- As a catalyst to prepare styrene derivatives from aryl halides and alkenes by Mizoroki-Heck reaction.
tert-Butoxide-Assisted Amidation of Esters under Green Conditions
Potassium tert-butoxide may be used as a base in the intramolecular cyclization of iodo arene to afford benzopyran via microwave method of synthesis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 2 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 156671-25G | 4061838742858 |
| 156671-25KG | 4061833509173 |
| 156671-5G | 4061833410028 |
| 156671-100G | 4061838742834 |
| 156671-10KG | |
| 156671-2.5KG | 4061838742841 |
| 156671-500G | 4061838742865 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service