All Photos(1)
About This Item
Empirical Formula (Hill Notation):
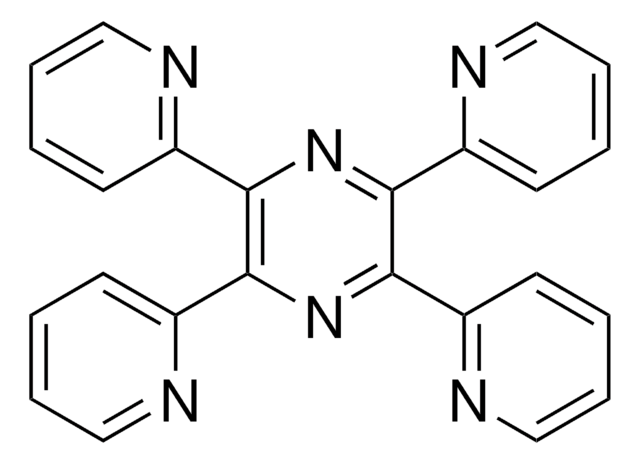
C20H16N4
CAS Number:
Molecular Weight:
312.37
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
191-193 °C (lit.)
SMILES string
Cc1cc2nc(-c3ccccn3)c(nc2cc1C)-c4ccccn4
InChI
1S/C20H16N4/c1-13-11-17-18(12-14(13)2)24-20(16-8-4-6-10-22-16)19(23-17)15-7-3-5-9-21-15/h3-12H,1-2H3
InChI key
NACXMBPTPBZQHY-UHFFFAOYSA-N
Application
6,7-Dimethyl-2,3-di-(2-pyridyl)quinoxaline has been used as an internal standard to investigate the clinical pharmacokinetics of nelfinavir mesylate, a potent inhibitor of HIV-1 protease.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Y Wu et al.
Journal of chromatography. B, Biomedical sciences and applications, 695(2), 373-380 (1997-08-01)
Nelfinavir mesylate, a potent and orally bioavailable inhibitor of HIV-1 protease (Ki=2 nM), has undergone Phase III clinical evaluation in a large population of HIV-positive patients. A high-performance liquid chromatography analytical method was developed to determine the pharmacokinetic parameters of
B Louveau et al.
Biomedical chromatography : BMC, 30(12), 2009-2015 (2016-06-10)
A precise and accurate high-performance liquid chromatography (HPLC) quantification method of rifampicin in human plasma was developed and validated using ultraviolet detection after an automatized solid-phase extraction. The method was validated with respect to selectivity, extraction recovery, linearity, intra- and
Sara Baldelli et al.
Therapeutic drug monitoring, 36(6), 739-745 (2014-04-18)
Recently, the European Medicines Agency (EMA) has released new guidelines on the validation of bioanalytical methods. In this work, we compared the analytical performance of 2 high-performance liquid chromatography with tandem mass spectrometry methods designed for the quantification of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service