114596
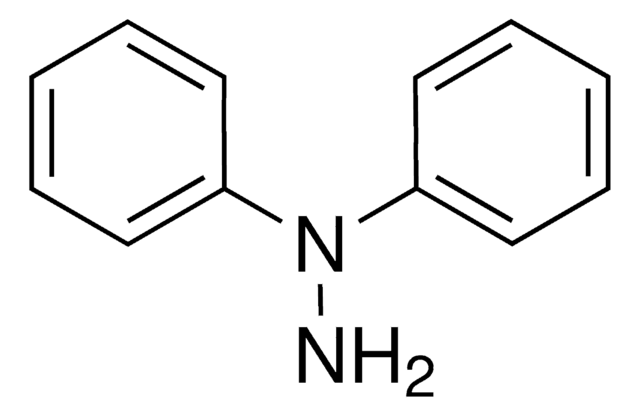
N,N-Diphenylhydrazine hydrochloride
97%
Synonym(s):
N,N-Diphenylhydrazine hydrochloride (asym.)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)2NNH2 · HCl
CAS Number:
Molecular Weight:
220.70
Beilstein:
3569340
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
SMILES string
Cl.NN(c1ccccc1)c2ccccc2
InChI
1S/C12H12N2.ClH/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12;/h1-10H,13H2;1H
InChI key
MIVUDWFNUOXEJM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N-Diphenylhydrazine hydrochloride is used in the synthesis of 2-Vinyloxyethyloxy-4-diethylaminophenyl-1-carbaldehyde N,N-diphenylhydrazone (hole-transporting hydrazine).
Other Notes
Small amounts of moisture cause melting point to vary greatly
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Formation of N-nitrosodiphenylamine from 1,1-diphenylhydrazine by rat liver microsomal preparations.
K Tatsumi et al.
Biochemical and biophysical research communications, 118(3), 958-963 (1984-02-14)
The present study provides the first evidence for in vitro metabolic conversion of a 1,1-disubstituted hydrazine to the corresponding nitrosamine. The study shows that superoxide radical which is generated by NADPH-cytochrome c reductase is involved in the oxidation of 1,1-diphenylhydrazine
New reactive hole-transporting hydrazone and its adducts with diol and dithiol.
Journal of Optoelectronics and Advanced Materials, 8(4), 1533-1533 (2006)
Reduction of N-nitrosodiphenylamine to the corresponding hydrazine by guinea pig liver preparations.
K Tatsumi et al.
Chemical & pharmaceutical bulletin, 30(10), 3842-3845 (1982-10-01)
João M S Cardoso et al.
Journal of inorganic biochemistry, 166, 55-63 (2016-11-12)
Camphorsulphonylimine complexes [Ag(NO
Sara S M Fernandes et al.
ACS omega, 3(10), 12893-12904 (2018-11-10)
A series of push-pull heterocyclic N,N-diphenylhydrazones were prepared to study the effect of structural modifications (different π-spacers and electron-withdrawing groups) on the optical (linear and nonlinear) and electronic properties of the molecules. The photovoltaic response of dye-sensitized solar cells assembled
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service