All Photos(1)
About This Item
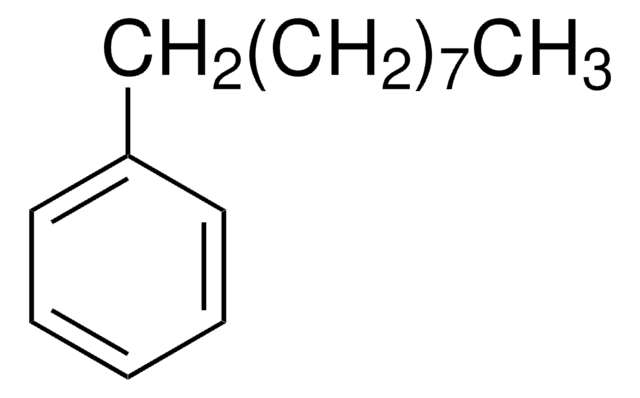
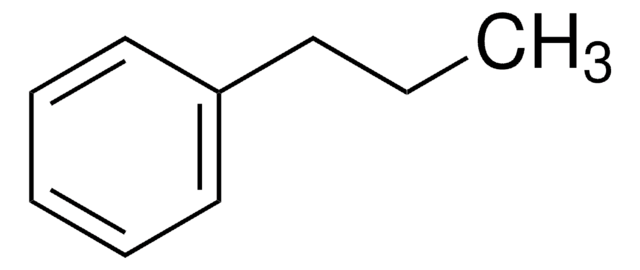
Linear Formula:
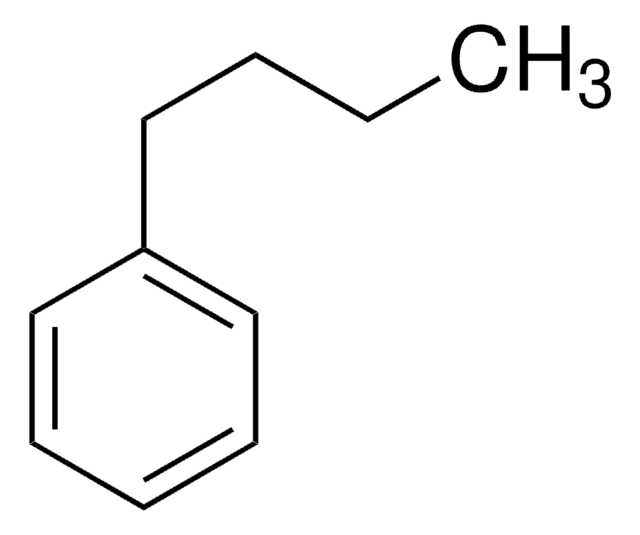
C6H5(CH2)7CH3
CAS Number:
Molecular Weight:
190.32
Beilstein:
1906253
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.484 (lit.)
bp
261-263 °C (lit.)
mp
−36 °C (lit.)
density
0.858 g/mL at 25 °C (lit.)
functional group
phenyl
SMILES string
CCCCCCCCc1ccccc1
InChI
1S/C14H22/c1-2-3-4-5-6-8-11-14-12-9-7-10-13-14/h7,9-10,12-13H,2-6,8,11H2,1H3
InChI key
CDKDZKXSXLNROY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Octylbenzene (1-Phenyloctane) forms charge-transfer complexes with fluoranil and 1,3,5-trinitrobenzene.
Application
1-Phenyloctane is used as a solvent to study the self-assembly of derivatives of tetrathiafulvalene (TTF) on graphite and their visualization by scanning tunnelling microscopy (STM). It is also used to study the effects of the substitution of these compounds on the interactions between them.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yanqiu Jiang et al.
Scientific reports, 5, 17720-17720 (2015-12-05)
The interface between organic semiconductor and graphene electrode, especially the structure of the first few molecular layers at the interface, is crucial for the device properties such as the charge transport in organic field effect transistors. In this work, we
Elba Gomar-Nadal et al.
Chemical communications (Cambridge, England), (7)(7), 906-907 (2003-05-13)
The synthesis, isolation and STM imaging on graphite of the cis and trans isomers of a TTF reveal isomer-dependent packing, and constitutes a way to study the non-covalent interactions at play in these systems.
Yan Yang et al.
Langmuir : the ACS journal of surfaces and colloids, 31(45), 12408-12416 (2015-10-29)
Side chains containing two diacetylene units spaced by an odd number of methylene units exhibit pronounced “bumps” composed of 0.3 nm steps, in opposite directions, at odd and even side-chain positions. In densely packed self-assembled monolayers, the bis-diacetylene bumps stack
Xiaodong Chen et al.
Nano letters, 7(11), 3483-3488 (2007-10-05)
Selective adsorption of semiconductor nanocrystals onto an organic self-organized pattern shows a time-dependent behavior. By studying the wetting behavior of delivered solvent (1-phenyloctane) on a lipid self-organized pattern and determining the adhesion energy between semiconductor nanocrystals and substrate, we obtain
Assemblies at the liquid-solid interface: chirality expression from molecular conformers.
Yong-Tao Shen et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 14(1), 92-95 (2012-11-13)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service