103136
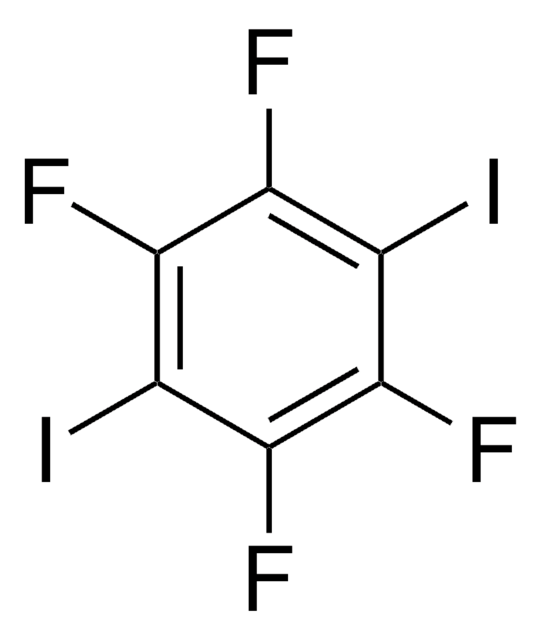
Iodopentafluorobenzene
99%
Synonym(s):
2,3,4,5,6-Pentafluoroiodobenzene, Pentafluoroiodobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6F5I
CAS Number:
Molecular Weight:
293.96
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.496 (lit.)
bp
161 °C (lit.)
density
2.204 g/mL at 25 °C (lit.)
functional group
fluoro
iodo
SMILES string
Fc1c(F)c(F)c(I)c(F)c1F
InChI
1S/C6F5I/c7-1-2(8)4(10)6(12)5(11)3(1)9
InChI key
OPYHNLNYCRZOGY-UHFFFAOYSA-N
General description
Iodopentafluorobenzene forms supramolecular complexes with aromatic electron donors by forming halogen bonds to form discrete heterodimeric aggregates.
Application
- Iodopentafluorobenzene was used to study the formation of radical ions of iodopentafluorobenzene in aqueous solution.
- It was used as solvent in a study to determine singlet oxgen lifetimes from phosphorescence decays in halogen substituted perfluorinated solvents by infrared emission spectrometery.
- It has potential applications in plasma processing industry and in preparation of catalysts.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Formation and redox properties of radical ions of iodopentafluorobenzene in aqueous solution: a pulse radiolysis study
Mohan H and Mittal JP
The Journal of Physical Chemistry, 99(33), 12559-12564 (1995)
Iodopentafluorobenzene: Electronic state spectroscopy by high-resolution vacuum ultraviolet photoabsorption and photoelectron spectroscopy.
Eden S, et al.
Chemical Physics, 359(1), 111-117 (2009)
Chenxin Ran et al.
Chemical Society reviews, 47(12), 4581-4610 (2018-04-24)
The rapid development of solar cells (SCs) based on organic-inorganic hybrid metal triiodide perovskite (MTP) materials holds great promise for next-generation photovoltaic devices. The demonstrated power conversion efficiency of the SCs based on MTP (PSCs for short) has reached over
Co-crystals of iodopentafluorobenzene with nitrogen donors: 2-D molecular assemblies through halogen bonding and aryl-perfluoroaryl interactions.
Wasilewska A, et al.
CrystEngComm, 9(3), 203-206 (2007)
Influence of heavy atoms on the deactivation of singlet oxygen (1. DELTA. g) in solution
Schmidt R
Journal of the American Chemical Society, 111(18), 6983-6987 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[Bis(trifluoroacetoxy)iodo]benzene purum, ≥95.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)