Ionic Liquids for Electrochemical Applications
Ionic liquids have attracted much interest for their use as non-aqueous electrolytes in electrochemical applications. In this context, their conductivity and electrochemical stability are the most important physical properties. Together with other properties, such as their negligible vapor pressure and non-flammability, they appear to be ideal electrolytes for many applications as described and discussed in a growing number of publications.1
Conductivity
Typical conductivity values are in the range from 1.0 mS/cm to 10.0 mS/cm. Recently, materials with conductivities above 20 mS/cm based on the imidazolium-cation were described: 1-ethyl-3-methylimidazolium thio-cyanate (Product No. 07424) and 1-ethyl-3-methylimidazolium dicyanamide (Product No. 00796).
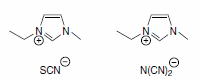
Of course, a solution of a typical inorganic salt such as sodium chloride in water has a higher conductivity. But, if we compare other properties of this solution with an ionic liquids, significant disadvantages become obvious: aqueous electrolytes are liquid over a smaller temperature range and the solvent water is volatile.
Electrochemical Stability
Another important property of ionic liquids is their wide electro-chemical window, which is a measure for their electrochemical stability against oxidation and reduction processes:
The electrochemical window is sensitive to impurities: halides are oxidized much easier than molecular anions (e.g., stable fluorine-containing anions such as bis(trifluoromethylsulfonyl)imide), where the negative charge is delocalized over larger volume. As a consequence, contamination with halides leads to significantly lower electrochemical stabilities.
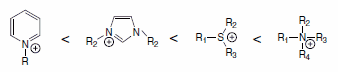
Cation Stability.
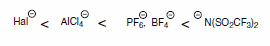
Anion Stability.
Conductivities and Electrochemical Windows |
|---|
Ionic Liquids Applications
High Conductivity
The materials showing the highest conductivities, 1-ethyl-3-methylimi-dazolium thiocyanate and dicyanamide exhibited the lowest electro-chemical stabilities. Nevertheless, these materials are good candidates for use in any application where a high conductivity combined with thermal stability and non-volatility is necessary, e.g., 1-dodecyl-3-methylimidazolium iodide (Product No. 18289) in dye-sensitized solar cells.2
High Stability
The electrochemically most stable materials having comparable small conductivities (N-butyl-N-methylpyrrolidinium bis(trifluoromethyl-sulfonyl)imide (Product No. 40963), triethylsulphonium bis(trifluoromethyl-sulfonyl)imide (Product No. 08748), and N-methyl-N-trioctylammonium bis(trifluoromethylsulfonyl)imide (Product No. 00797). These materials are good electrolytes for use in batteries,3 fuel cells,4 metal deposition,5 and electrochemical synthesis of nano-particles.6
Combined Properties
For applications where conductivity and electrochemical stability are needed (e.g., supercapacitors7 or sensors8), imidazolium-based ionic liquids with stable anions (e.g., tetrafluoroborate or trifluoromethylsulfonate) are the materials of choice.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?