Cell Culture Fundamentals: Cryopreservation and Storage of Cell Lines
ECACC Laboratory Handbook 4th Edition
Cryopreservation of Cell Lines
The aim of cryopreservation, or cell freezing, is to enable stocks of cells to be stored to prevent the need to have all
cell lines in culture at all times. It is invaluable when dealing with cells of limited life span. The other main advantages of cryopreservation are:
- Reduced risk of microbial contamination
- Reduced risk of cross contamination with other cell lines
- Reduced risk of genetic drift and morphological changes
- Work conducted using cells at a consistent passage number
- (refer to section 8 ‘Good Cell Banking Practices’)
- Reduced costs (consumables and staff time)
There has been a large amount of developmental work undertaken to ensure successful cryopreservation and resuscitation of a wide variety of cell lines of different cell types. The basic principle of successful cryopreservation and resuscitation is a slow freeze and quick thaw. Although the requirements may vary amongst cell lines, as a general guide cells should be cooled at a rate of –1 °C to –3°C per minute and thawed quickly by incubation in a 37 °C water bath for 3-5 minutes. If this and the additional points given below are followed, most cell lines should be cryopreserved successfully.
- Cultures should be healthy with a viability of >90% and no signs of microbial contamination.
- Cultures should be in log phase of growth (this can be achieved by using pre-confluent cultures that are below their maximum cell density and by changing the culture medium 24 hours before freezing).
- A high concentration of serum/protein (>20%) should be used. In many cases serum is used at 90%.
- Use a cryoprotectant such as dimethyl sulphoxide (DMSO) or glycerol to help protect the cells from rupture by the formation of ice crystals. The most commonly used cryoprotectant is DMSO at a final concentration of 10%. However, this is not appropriate for all cell lines such as when DMSO is used to induce differentiation. In such cases an alternative such as glycerol should be used (refer to ECACC data sheet for details of the correct cryoprotectant). We offer ready-made cell freezing media containing DMSO, glycerol and serum-free formulations containing DMSO.
- Slow freezing, decreasing the temperature approximately 1 °C per minute, using a Nalgene Mr. Frosty Freezing Container or Corning Cool Cell Freezing Container may aid in successful cell cryopreservation.
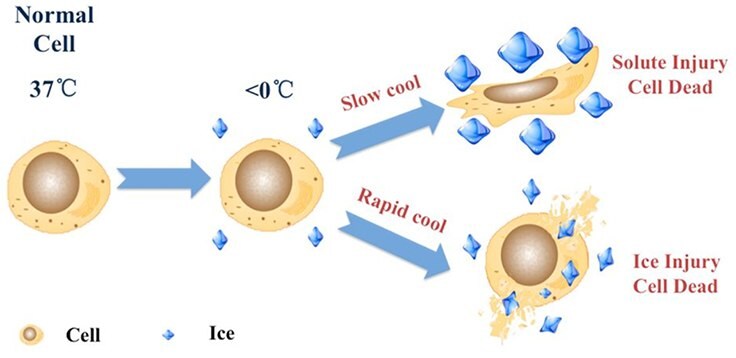
Figure 1. Cell cryopreservation mechanism. Cryoprotectants, such as DMSO or glycerol, may be added to cell culture media as a cryoprotectant for cells. DMSO reduces ice crystal formation and thereby prevents cell death during the freezing process. Approximately 10% v/v may be used with a slow-freeze method (decreasing the temperature approximately 1°C per minute) and the cells may be frozen at −80 °C (−112 °F) or stored in liquid nitrogen for extended periods of time.
Ultra-low Temperature Storage of Cell Lines
Following controlled rate freezing in the presence of cryoprotectants, cell lines can be cryopreserved in a suspended state for indefinite periods provided a temperature of less than -135 °C is maintained. Such ultra-low temperatures can only be attained by specialized electric freezers or more usually by immersion in liquid or vapor phase nitrogen. The advantages and disadvantages can be summarized as follows:
Storage in liquid phase nitrogen allows the lowest possible storage temperature to be maintained with absolute consistency, but requires the use of large volumes (depth) of liquid nitrogen which is a potential hazard. There have also been documented cases of cross contamination by virus pathogens via the liquid nitrogen medium. For these reasons ultra-low temperature storage is most commonly in vapor phase nitrogen.
For vapor phase nitrogen storage, the ampoules are positioned above a shallow reservoir of liquid nitrogen, the depth of which must be carefully maintained. A vertical temperature gradient will exist through the vapor phase, the extremes of which will depend on the liquid levels maintained, the design of the vessel, and the frequency with which it is opened. Temperature variations in the upper regions of a vapor phase storage vessel can be extreme if regular maintenance is not carried out. Modern designs of liquid nitrogen storage vessels are increasingly offering improved vapor phase storage technology.
Loss of entire cell stocks through inadequate storage maintenance is distressingly common. All liquid nitrogen storage vessels should minimally include alarms that warn of low liquid nitrogen levels and should also be constantly temperature monitored and alarmed. This is particularly true of vapor phase storage systems. The bulk liquid nitrogen storage vessel should not be allowed to become less than half full before it is resupplied. This will ensure that at least one liquid nitrogen delivery can be missed without catastrophic consequences. It is highly recommended that valuable cell stocks should be backed up by storage at a second site. ECACC offers a Safe Deposit Service for this purpose.

Figure 2. Common liquid nitrogen storage tanks used for cell cryopreservation.
Cell Line Inventory Control
All ultra-low temperature storage vessels should include a racking / inventory system designed to organize the contents for ease of location and retrieval of frozen cryovials. This should be supported by accurate record keeping and inventory control incorporating the following:
- Each ampoule should be individually labelled, using liquid nitrogen resistant labels with identity, lot number, passage number and date of freezing.
- The location of each ampoule should be recorded ideally on an electronic database or spreadsheet, but also on a paper storage plan.
- There should be a control system to ensure that no ampoule can be deposited or withdrawn without updating the records.
Liquid Nitrogen Safety Considerations
General Safety Issues
It is important that staff are trained in the use of liquid nitrogen and associated equipment including the storage vessels which need to be vented safely and containers which may need to be filled. As with all laboratory procedures personal protective equipment should be worn always whilst handling nitrogen, including a full-face visor and thermally insulated gloves in addition to a laboratory coat and preferably a splash proof plastic apron. Proper training and the use of protective equipment will minimize the risk of frostbite, burns and other adverse incidents.
Risk of Asphyxiation
The single most important safety consideration is the potential risk of asphyxiation when escaped nitrogen vaporizes and displaces atmospheric oxygen. This is critical since oxygen depletion can very rapidly cause loss of consciousness, without warning. Consequently, liquid nitrogen refrigerators should be placed in well-ventilated areas in order to minimize this risk and be subject to planned preventative maintenance. Large volume stores should have low oxygen alarm systems.

Precautions for Dedicated Liquid Nitrogen Storage Areas
- Use oxygen alarms set to 18% oxygen (v/v)
- Staff training – staff should be trained to evacuate the area immediately on hearing the alarm and not return until the oxygen is back to normal levels (~ 20% v/v)
- Staff should work in pairs when handling liquid nitrogen
- Prohibit the use of nitrogen outside of normal working hours
- Mechanical ventilation systems should be installed if possible
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?