Tissue-Specific Decellularized Extracellular Matrix (dECM) Hydrogels for Enhanced 3D Cell Cultures
- 3D cell culture models
- Decellularized extracellular matrix (dECM) scaffolds
- Lung dECM hydrogels
- Liver dECM hydrogels
- Bone dECM hydrogels
- dECM Gel Hydrogel kits
3D Cell Culture Models
The cellular microenvironment directly and indirectly effects cellular behavior via both biophysical and biochemical routes. The microenvironment is composed of extracellular matrix (ECM) proteins, surrounding cells, cytokines, growth factors, hormones, and other bioactive agents, possessing nano/microscale mechanical properties such as tension and stiffness. Traditional 3D cell cultures rely on encapsulating cells in undefined Engelbreth-Holm-Swarm (EHS) sarcoma-derived basement membrane (BME) extracts such as Matrigel® matrix (E1270). These hydrogels contain a heterogeneous mixture of both growth factors (TGFβ, EGF, IGF, etc.) and ECM proteins (laminins, collagens, entactin, etc.) at elevated concentrations. Additionally, these tumor-derived hydrogels do not fully recapitulate the native tissue microenvironment and can lead to variable and misleading experimental results. Recently, synthetic hydrogels have been used to grow cells in three dimensions. However, these hydrogels often lack the biological complexity and functionality needed to fully mimic the native tissue microenvironment for 3D cultures.
Decellularized Extracellular Matrix (dECM) Scaffolds
To isolate the native extracellular matrix of a tissue from its inhabiting cells, a decellularization process is employed. The resulting decellularized extracellular matrix (dECM) scaffolds can be used in cell culture and tissue engineering applications. Compared to other tumor-derived basement membrane extracts (e.g., Matrigel® matrix), these dECM hydrogel scaffolds can provide a more physiological environment with increased growth rates for cells without the use of any exogenous growth factors.
Figure 1. Tissue decellularization overview. Porcine derived organs (liver, lung, heart, etc.) were decellularized and the native tissue specific ECM components were solubilized and frozen for future use. These dECM hydrogels can be used for traditional 2D ECM coatings or to encapsulate cells for 3D cell cultures.
Our tissue-specific dECM Gel Hydrogel Kits are a series of native decellularized extracellular matrices (dECMs) derived from a variety of non-diseased porcine organs (bone, heart, liver, kidney, intestine, skin and lung). These hydrogels can be used as traditional 2D ECM coatings or to encapsulate cells for 3D cell culture applications, with enhanced performance compared to other hydrogels.
Lung dECM Hydrogels for 3D Cell Culture
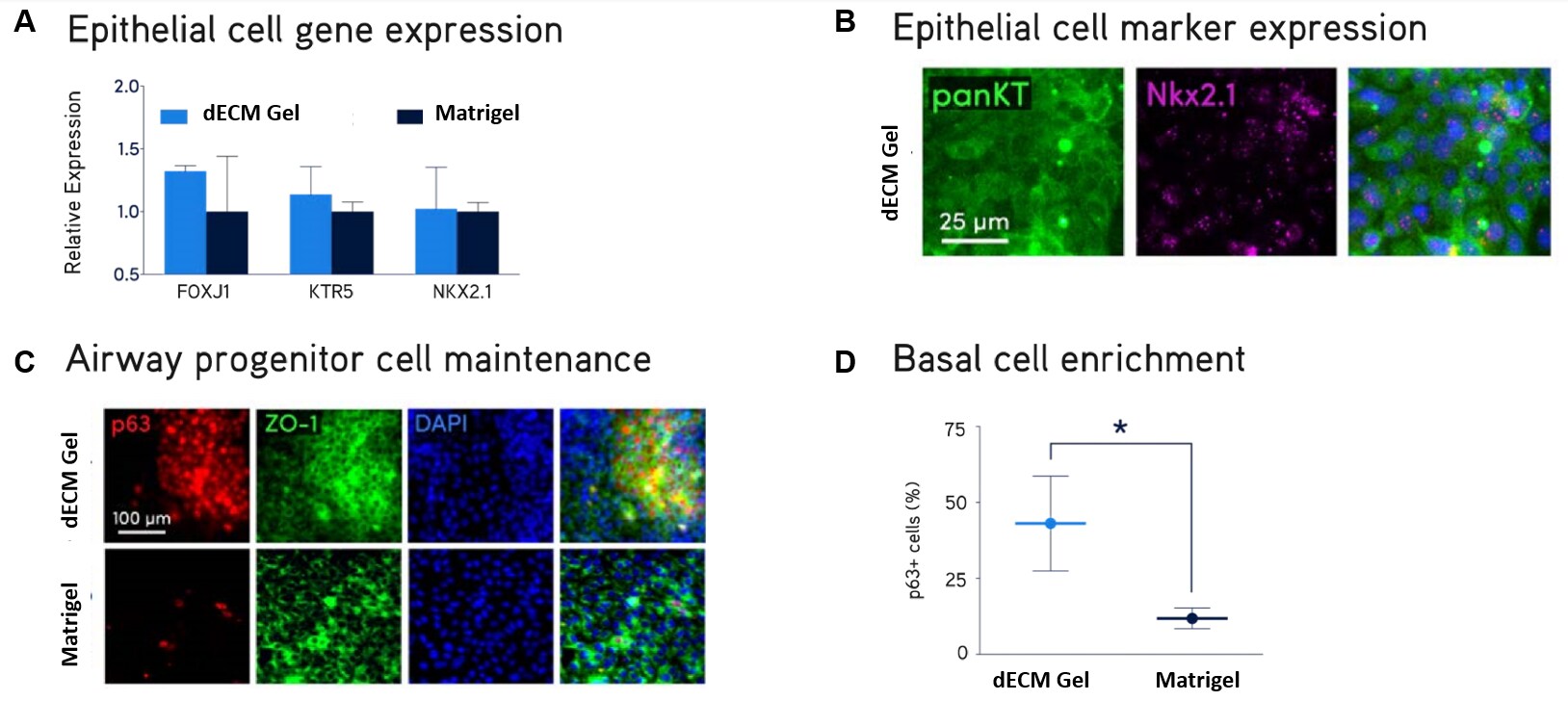
Figure 2. Primary NHBE cell culture.Primary normal human bronchial epithelial (NHBE) cells were cultured on Lung dECM Hydrogels or Matrigel® matrix for 10 days. NHBE cells cultured on dECM Hydrogels supported robust expression of normal lung epithelial cell markers FOXJ1, KTR5, NKX2.1, panKT, p63 and ZO-1 (A,B,C) and generated a larger subpopulation of p63+ basal airway cells (D) compared to Matrigel® matrix
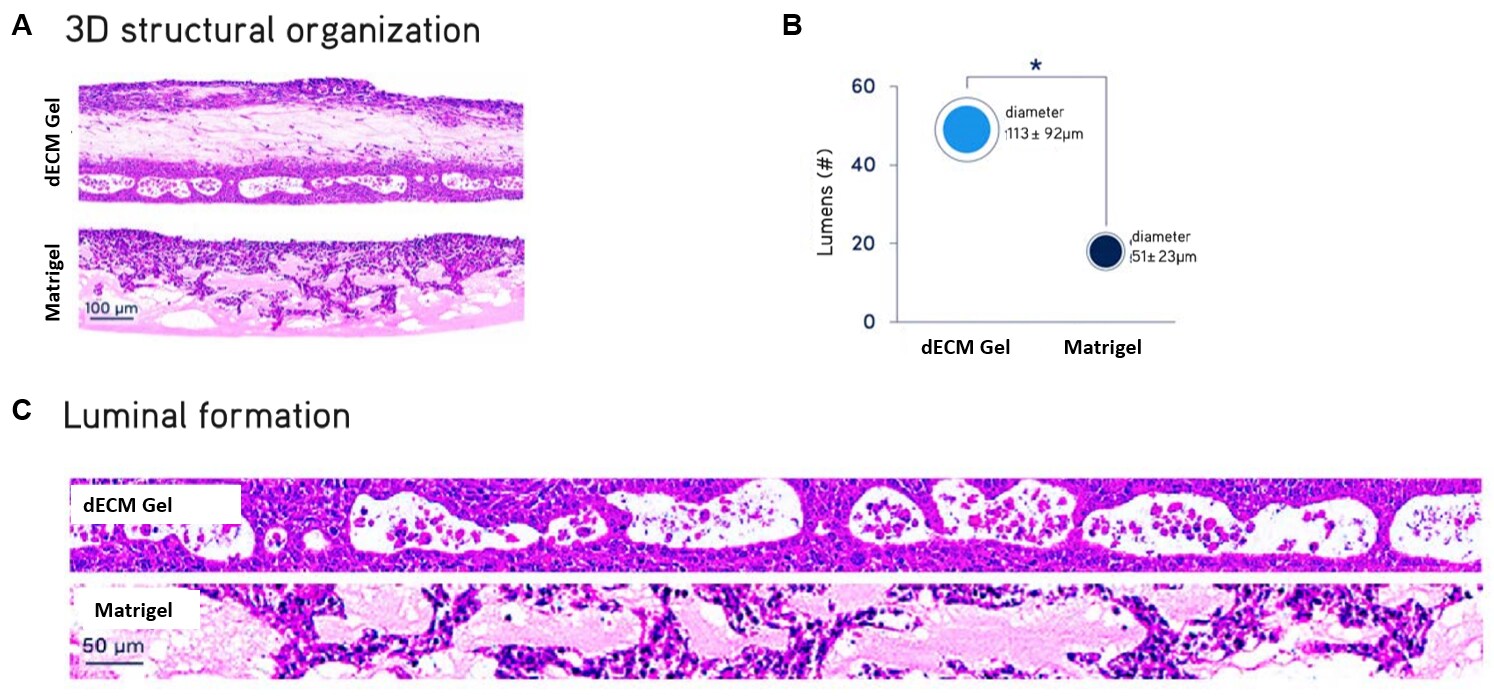
Figure 3. ALI cultures of primary NHBE cells.Air liquid interface (ALI) cultures of primary normal human bronchial epithelial (NHBE) cells after 21 days. NHBE cells cultured in Lung dECM Hydrogels formed more organized and complex stratified luminal structures recapitulating the cellular architecture of the human airway, with significantly larger average diameter compared to Matrigel® matrix.
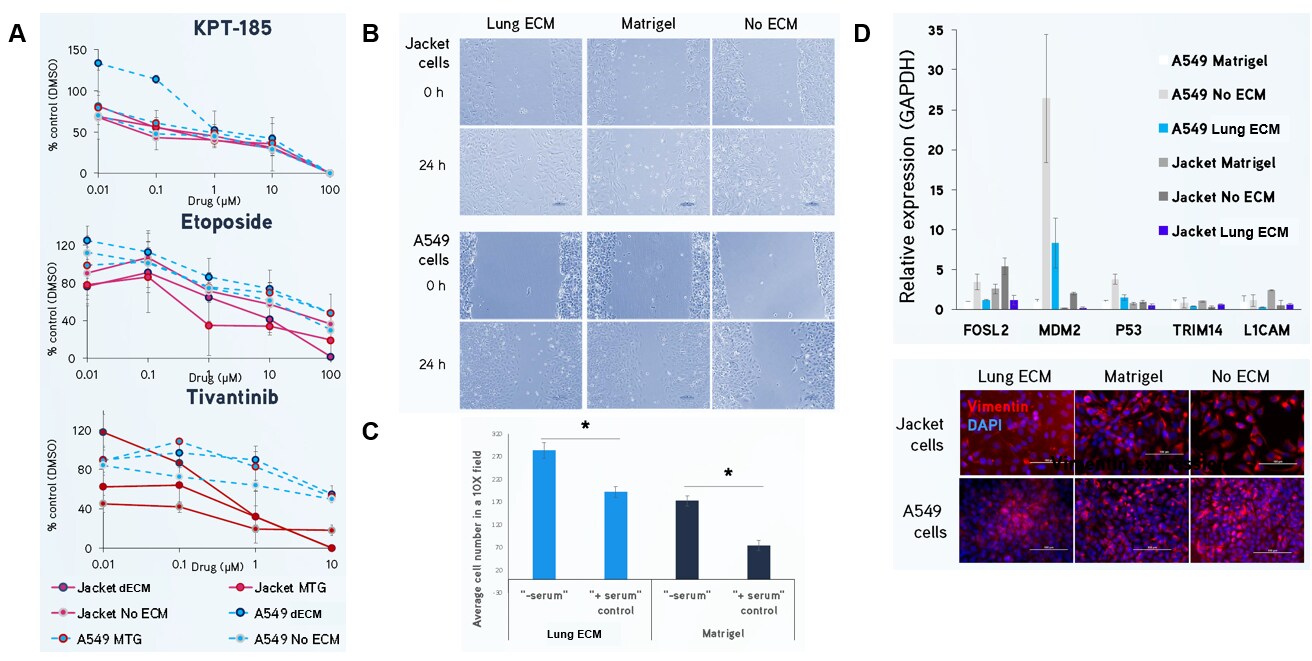
Figure 4. Lung dECM Hydrogels can be used to model human lung cancer.(A) Jacket and A549 lung adenocarcinoma cells were exposed to KPT-185, etoposide and tivantinib for 72 hours and cell number was analyzed using a MTT assay. Cells cultured in Lung dECM Hydrogel exhibited distinct drug resistance profiles with higher IC50 values compared to Matrigel® matrix, which may indicate a more predictive physiological response. (B, C) Jacket and A549 lung adenocarcinoma cells display increased migration and invasion after 24 hours using scratch and Boyden chamber assays when cultured on Lung dECM Hydrogels. (D) Gene expression of Jacket and A549 cultured in Lung dECM hydrogels. Cells cultured in Lung dECM Hydrogels exhibited lower cancer-related gene expression than cells cultured in Matrigel® matrix including lower vimentin expression.
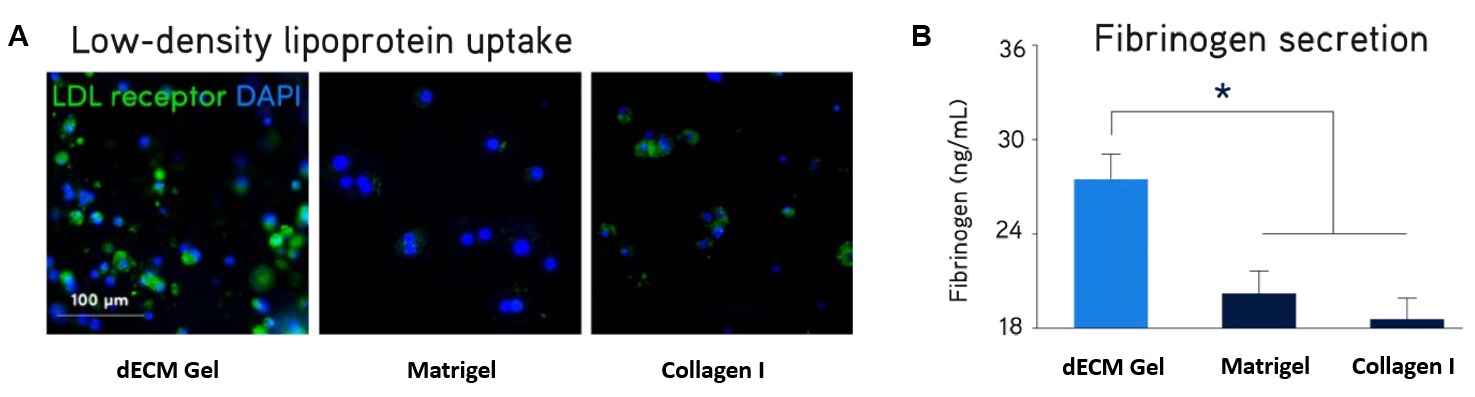
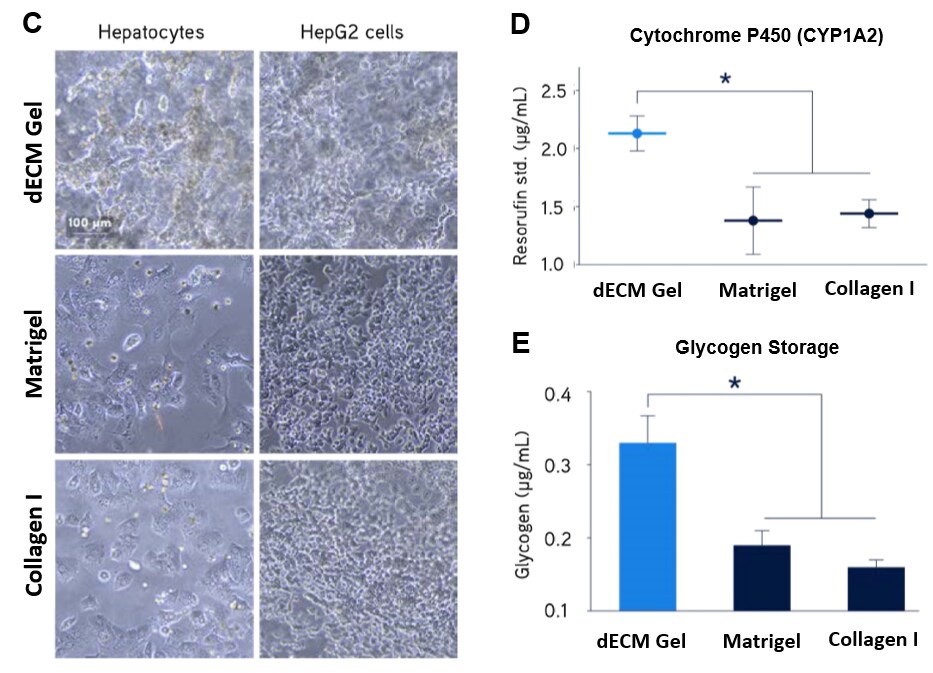
Figure 5. Primary human hepatocyte culture.Primary human hepatocytes showed significantly higher expression of LDL receptor (A) and secretion of fibrinogen (B) when cultured in Liver dECM Hydrogels compared to Matrigel® matrix or Collagen I. Primary human hepatocytes and HepG2 hepatocarcinoma liver cells showed significantly higher 3D structure formation (C), enhanced cytochrome P450 activity (D), and increased glycogen storage (E) when cultured in Liver dECM Hydrogels compared to Matrigel® matrix or Collagen I.
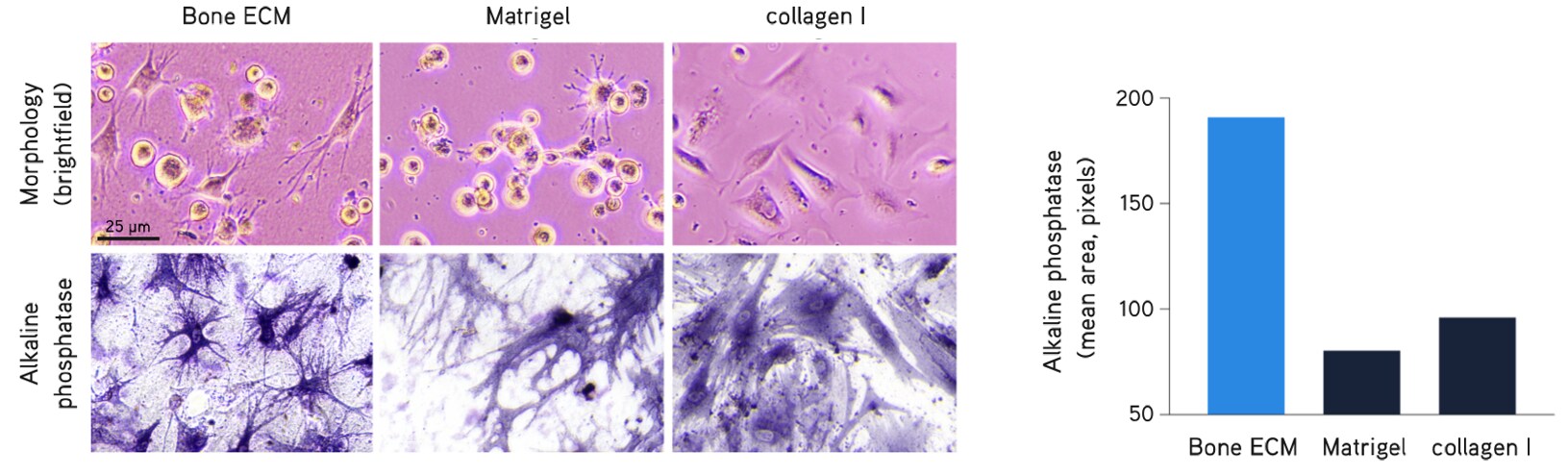
Figure 6. Primary human osteoblasts culture.Primary human osteoblasts cultured in Bone dECM Hydrogel (6 mg/mL) show characteristic dendritic morphology after 1 hour in culture. Osteoblasts displayed higher alkaline phosphatase activity in Bone dECM Hydrogels than in Matrigel® matrix or collagen I after 7 days in culture.
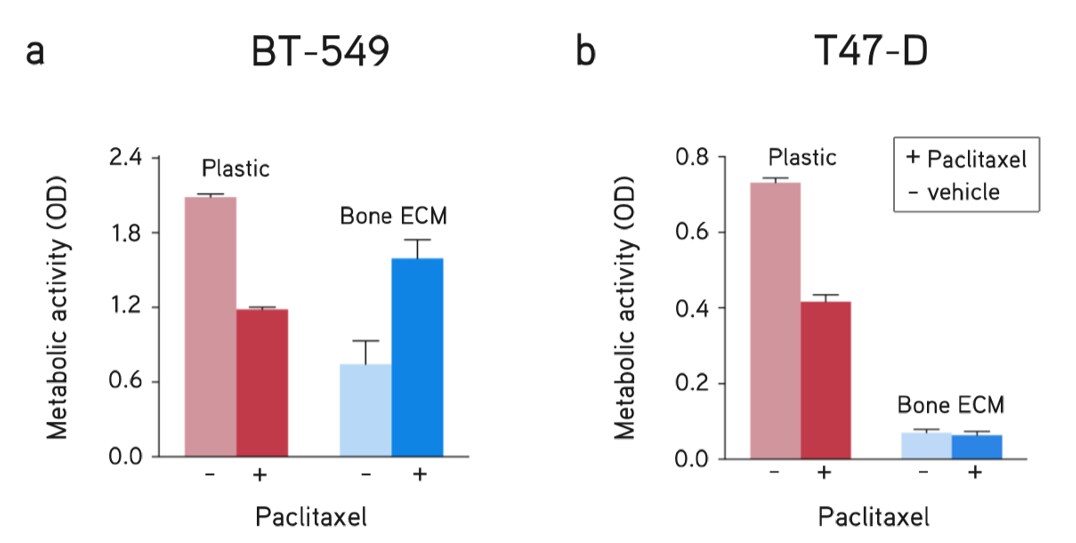
Figure 7. Breast cancer metastasis assay. dECM Hydrogels utilized as a model of breast cancer metastasis to bone showed differential drug responses of breast cancer subtypes compared to culturing on plastic (no ECM). Response to drug treatment (paclitaxel, 5 µM) and vehicle (DMSO) of (A) BT-549 cells and (B) T47-D cells for 48 hours.
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?