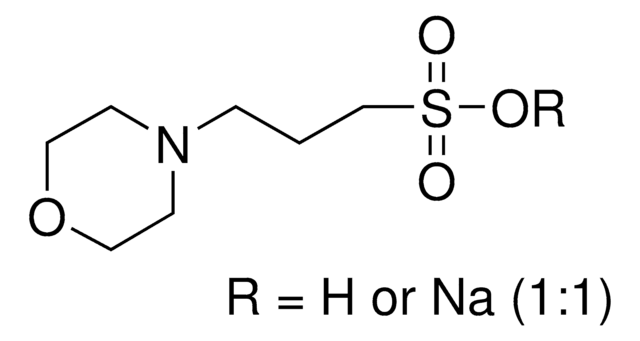
T9269
TAPSO
≥99% (titration)
Sinónimos:
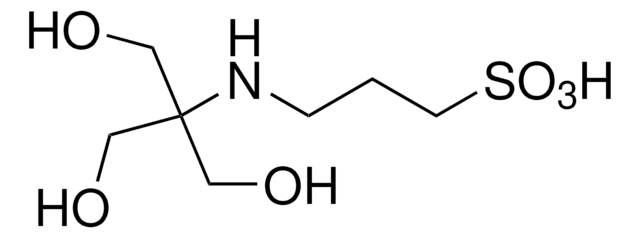
2-Hydroxy-3-[tris(hydroxymethyl)methylamino]-1-propanesulfonic acid, N-[Tris(hydroxymethyl)methyl]-3-amino-2-hydroxypropanesulfonic acid
About This Item
Productos recomendados
Quality Level
assay
≥99% (titration)
form
crystalline powder
useful pH range
7.0-8.2
pKa (25 °C)
7.6
solubility
warm water: 0.333 g/mL, clear to slightly hazy, colorless
application(s)
diagnostic assay manufacturing
SMILES string
OCC(CO)(CO)NCC(O)CS(O)(=O)=O
InChI
1S/C7H17NO7S/c9-3-7(4-10,5-11)8-1-6(12)2-16(13,14)15/h6,8-12H,1-5H2,(H,13,14,15)
InChI key
RZQXOGQSPBYUKH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Interpretation of non-Nernstian slopes in graphic analysis of data collected in pH range close to deprotonation of a ligand Part I. A glass electrode potentiometric and polarographic study of Cd-(TAPSO)x-(OH)y and Zn-(TAPSO)x-(OH)y systems.: Explores the complex behavior of TAPSO in metal binding studies, particularly with cadmium and zinc, providing insight into its utility in heavy metal chelation and environmental cleanup strategies (Machado et al., 2006).
- Challenges in modelling and optimisation of stability constants in the study of Cu-(TAPS)(x)-(OH)(y) system by polarography.: Discusses the challenges in modeling and optimizing the stability constants for copper complexes with TAPSO, contributing to better understanding and utilization of this buffer in copper-related biochemical research (Machado and Soares, 2007).
- Modelling of Pb-(TAPS)(x)-(OH)(y) system and refinement of stability constants in the region of lead hydrolysis and lead hydroxide precipitation.: Provides a detailed examination of TAPSO′s role in modeling lead complexation systems, which is critical for environmental monitoring and remediation efforts involving lead (Machado et al., 2007).
- Synthetic organic pH buffers can support fertilization of guinea pig eggs, but not as efficiently as bicarbonate buffer.: Evaluates the efficacy of synthetic buffers like TAPSO in supporting fertilization, offering valuable data for reproductive biology and assisted reproductive technologies (Bhattacharyya and Yanagimachi, 1988).
Caution
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico