T2879
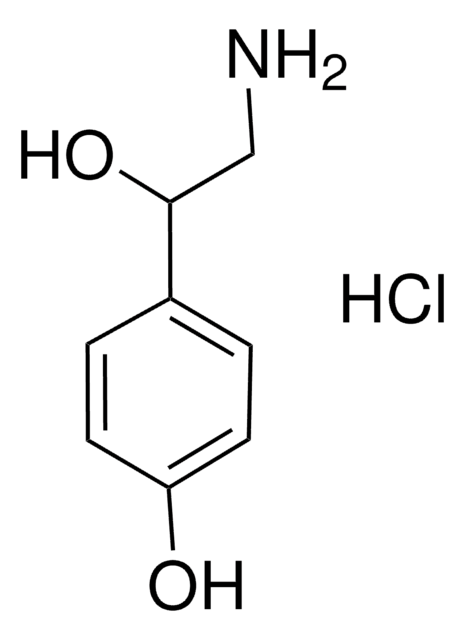
Tyramine hydrochloride
≥98% (TLC), powder, neuromodulator
Sinónimos:
4-(2-Aminoethyl)phenol hydrochloride, 4-Hydroxyphenethylamine hydrochloride, Tyrosamine hydrochloride
About This Item
Productos recomendados
Nombre del producto
Tyramine hydrochloride, ≥98%
Quality Level
assay
≥98%
mp
271-274 °C (lit.)
solubility
H2O: soluble 50 mg/ml, clear, colorless to faintly yellow
SMILES string
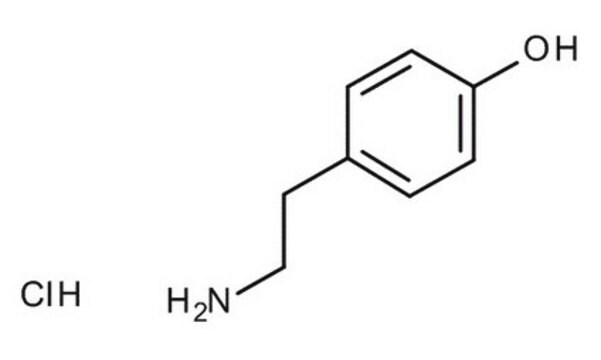
Cl[H].NCCc1ccc(O)cc1
InChI
1S/C8H11NO.ClH/c9-6-5-7-1-3-8(10)4-2-7;/h1-4,10H,5-6,9H2;1H
InChI key
RNISDHSYKZAWOK-UHFFFAOYSA-N
Gene Information
human ... ADORA2A(135) , ADORA2B(136) , ADORA3(140)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- coinfused with adenosine in control subjects and patients in order to reduce leg blood flow by 50% without affecting arterial blood pressure
- labelled with fluorescence dyes (Atto 488 and Atto 655) to serve as a substrate for peroxidase in immunofluorescence analysis
- used in dimethylformamide, labelled with 5-(and-6)carboxyfluorescein, succinimidyl ester/biotin to serve as a substrate for peroxidase in tyramide signal amplification
Biochem/physiol Actions
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico