S4316
Amyloid Precursor Protein β, Secreted human
recombinant, expressed in E. coli (N-terminal histidine tagged), solution
Sinónimos:
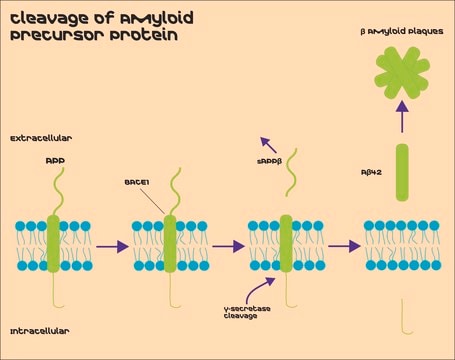
Amyloid Precursor Protein β, Secreted, sAPPβ
About This Item
Productos recomendados
General description
Application
Biochem/physiol Actions
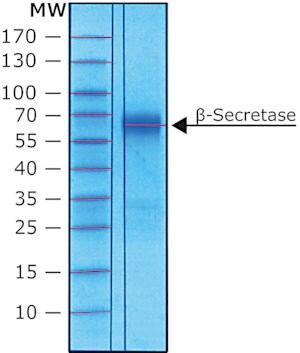
Physical form
Preparation Note
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Central Nervous System of Humans and Rhesus
Macaques
Artículos
Alzheimer's disease (AD) is the most common cause of dementia in the elderly and is characterized by gradual loss of cognitive functions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico