O0382
Anti-Opioid δ Receptor antibody produced in rabbit
whole antiserum, lyophilized powder
About This Item
Productos recomendados
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
whole antiserum
antibody product type
primary antibodies
clone
polyclonal
form
lyophilized powder
mol wt
antigen ~55 kDa
species reactivity
mouse (predicted), rat
technique(s)
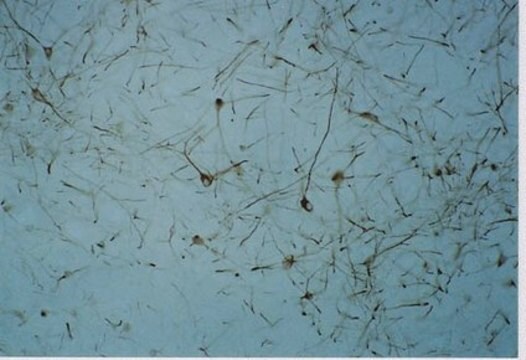
immunocytochemistry: 1:800 using PLP fixed rat brain sections
western blot: 1:800 using whole brain homogenate
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
mouse ... Oprd1(18386)
rat ... Oprd1(24613)
General description
δ-Opioid receptors (DOR) are located postsynaptically on pallidostriatal feedback neurons. DORs also modulate nociception presynaptically in the periaqueductal gray where immunolabeling of DOR has been shown to be intracellular and often associated with large dense-core vesicles. Additionally, receptor autoradiographic investigations have localized DORs to the external plexiform layer of the olfactory bulb, the nucleus accumbens, several layers of the cerebral cortex and several nuclei of the amygdala.
References
1. Goldstein, A. Trends Pharmacol. Sci., 8, 456-459 (1987).
Specificity
Immunogen
Disclaimer
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico