MSQC3
SILu™MAB Stable-Isotope Labeled Universal Monoclonal Antibody Standard human
recombinant, expressed in CHO cells
Sinónimos:
Mass spectrometry standard, IgG, Mass spectrometry standard, Immunoglobulin-G, Stable isotope labelled IgG
About This Item
Productos recomendados
recombinant
expressed in CHO cells
Quality Level
assay
≥90% (SDS-PAGE)
packaging
vial of 100 μg (± 10% Lot-specific vial content given on certificate of analysis)
shipped in
wet ice
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
Features and Benefits
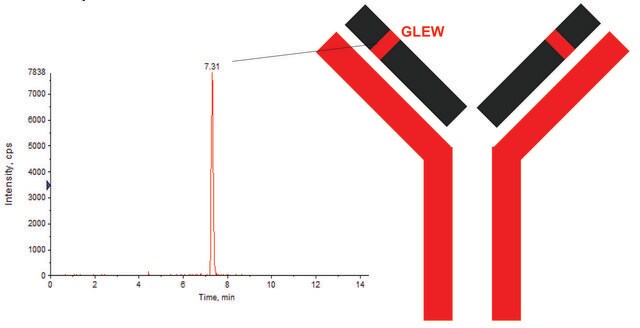
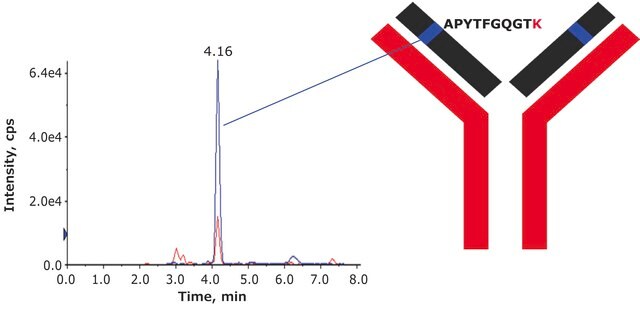
DTLMISRHeavy Chain (IgG1, IgG2, IgG3, IgG4)
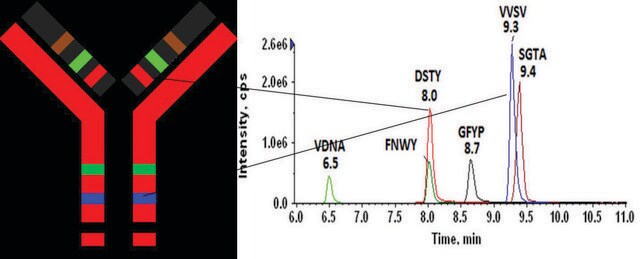
FNWYVDGVEVHNAKHeavy Chain (IgG1)
VVSVLTVLHQDWLNGKHeavy Chain (IgG1, IgG3, IgG4)
NQVSLTCLVKHeavy Chain (IgG1, IgG2, IgG3, IgG4)
GFYPSDIAVEWESNGQPENNYKHeavy Chain (IgG1, IgG4)
AGVETTTPSKLight Chain (lambda)
YAASSYLSLTPEQWKLight Chain (lambda)
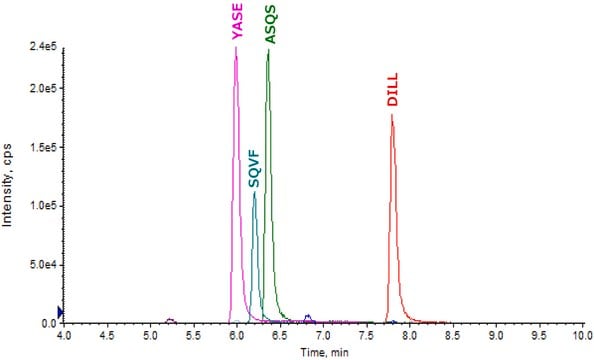
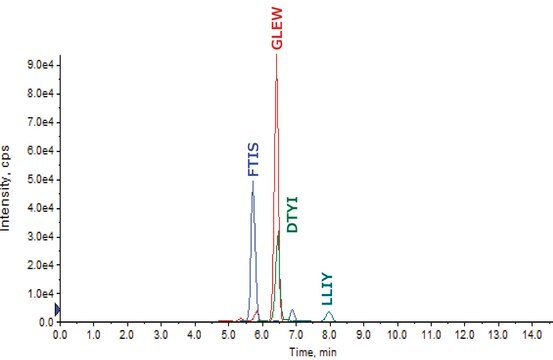
- SILuMab yielded reproducible, linear curves from 0.1 μg/mL to 1000 μg/mL without enrichment or depletion.
- Good agreement was observed between multiple peptides derived from the same target.
- Label incorporation was determined to be >98% by mass spectrometry.
- Sequence coverage was confirmed by peptide mapping.
Physical form
Preparation Note
Reconstitution
Procedure
- Briefly centrifuge the vial at ~10,000 x g to collect the product at the bottom of the vial.
- Add 500 μL of purified water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for a minimum of 15 minutes and repeat mixing by inversion.
Analysis Note
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SILuMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
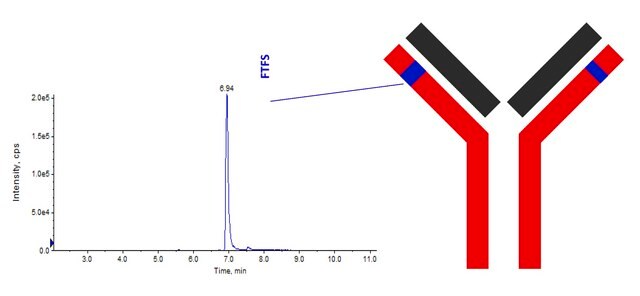
Target overlap areas are underlined
Intact heavy and light chains (FASTA file)
Quantitative
MRM settings provided (Skyline, xls)
Legal Information
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico