F0162
Fibronectina
lyophilized powder, 45 kDa
Sinónimos:
Fibronectin
About This Item
Productos recomendados
biological source
human plasma
Quality Level
assay
≥90% (SDS-PAGE)
form
lyophilized powder
mol wt
45 kDa
packaging
pkg of 0.5 mg
technique(s)
cell culture | mammalian: suitable
impurities
HIV and HBsAg, source material tested negative
Small proteolytic fragments, may contain traces
solubility
water: soluble ≥0.500 mg/mL, clear to slightly hazy, colorless
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... FN1(2335)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
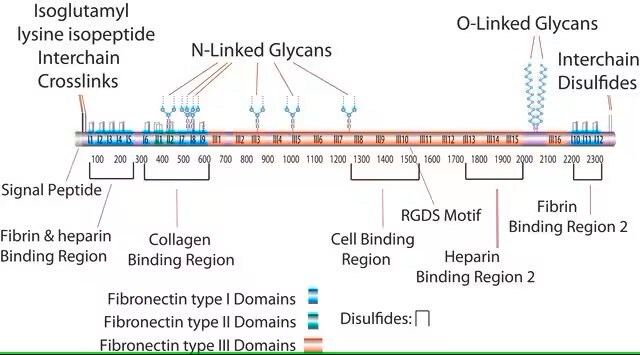
This fragment has an acidic pI (4.9-5.3) and does not bind to heparin. This domain is resistant to proteolysis due to intrachain disulfide bonding and the attached carbohydrate. The intrachain disulfide bonds are essential for binding to gelatin, while the complex, branched, asparagine-linked carbohydrate is not. This fragment binds to C1q, but not to fibrin.
Caution
Preparation Note
Optional
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Extracellular matrix proteins such as laminin, collagen, and fibronectin can be used as cell attachment substrates in cell culture.
Protocolos
Dilute fibronectin to the desired concentration. Optimum conditions for attachment are dependent on cell type and application. The typical coating concentration is 1 – 5 ug/cm2.Fibronectin coating protocol, products, and FAQs at sigmaaldrich.com
Dilute fibronectin to the desired concentration. Optimum conditions for attachment are dependent on cell type and application. The typical coating concentration is 1 – 5 ug/cm2.Fibronectin coating protocol, products, and FAQs.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico