A9611
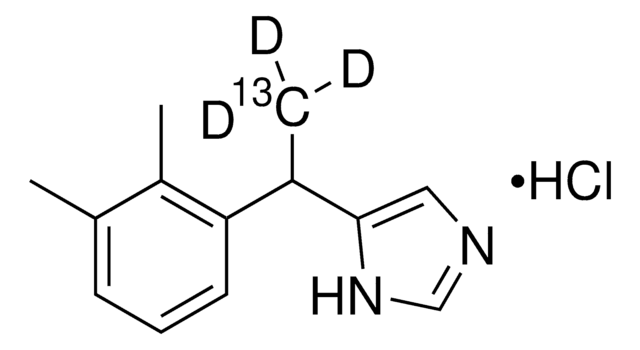
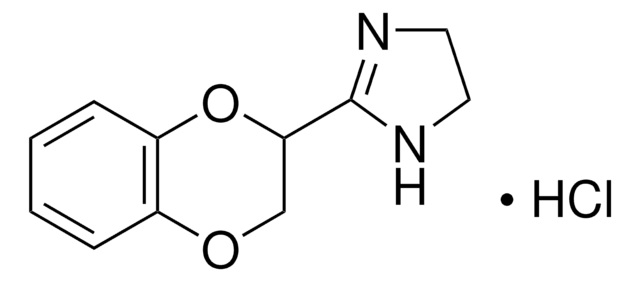
Atipamezole
≥98% (HPLC)
Sinónimos:
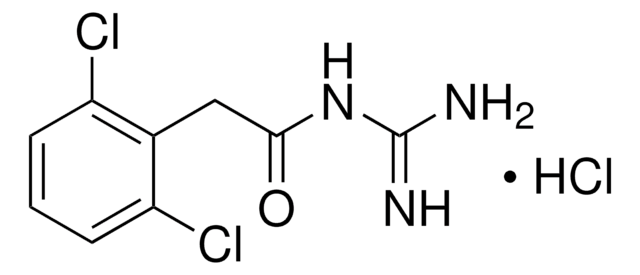
4-(2-Ethyl-2,3-dihydro-1H-inden-2-yl)-1H-Imidazole, Antisedan, MPV 1248
About This Item
Productos recomendados
Quality Level
assay
≥98% (HPLC)
form
powder
color
white to brown
solubility
DMSO: ≥30 mg/mL
storage temp.
room temp
SMILES string
CCC1(Cc2ccccc2C1)c3c[nH]cn3
InChI
1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16)
InChI key
HSWPZIDYAHLZDD-UHFFFAOYSA-N
General description
Application
Biochem/physiol Actions
Features and Benefits
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
α2-Adrenoceptors
Contenido relacionado
DISCOVER Bioactive Small Molecules for Neuroscience
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico