A8312
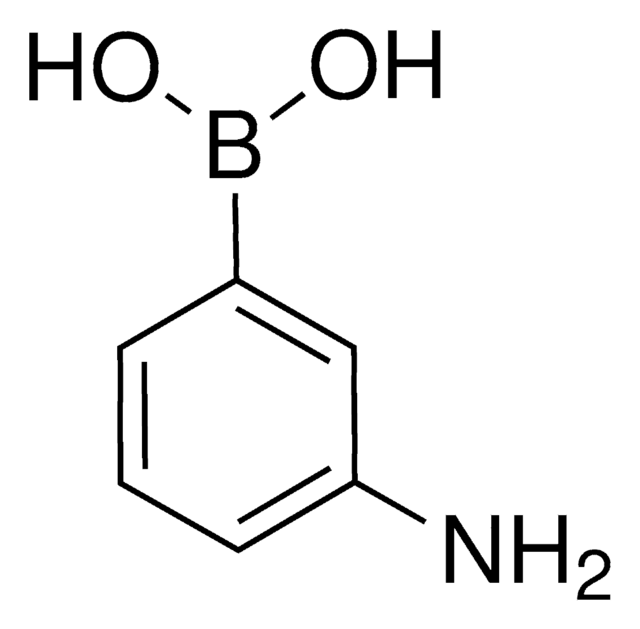
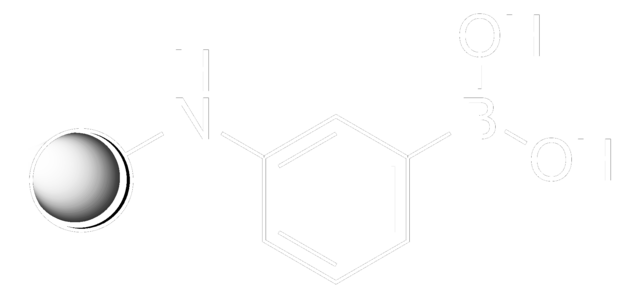
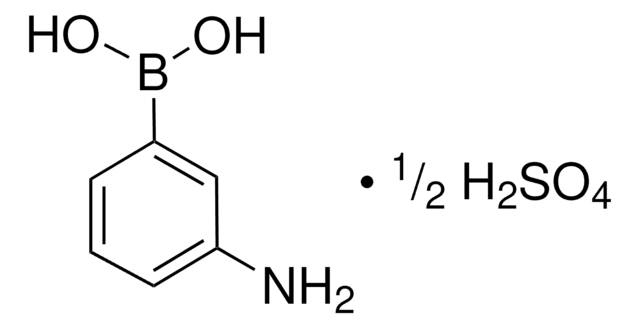
m-Aminophenylboronic acid–Agarose
aqueous suspension
Sinónimos:
(3-aminophenyl)boronic acid-Agarose, 3-Aminophenylboronic acid–Agarose, m-APBA-Agarose, m-Aminophenylboronic acid resin
About This Item
Productos recomendados
form
aqueous suspension
Quality Level
extent of labeling
40-80 μmol per mL
matrix
6% beaded agarose
matrix activation
epichlorohydrin
matrix attachment
through amino to carboxyls of EDTA
matrix spacer
9 atoms
capacity
≥8 mg/mL binding capacity (peroxidase Type VI)
storage temp.
2-8°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as a component of the column for the purification of Escherichia coli TOP10F cells
- with cell lysates of various cells for detection of PARylated (Poly(ADP-ribos)ylation) proteins.
- in the affinity purification of horseradish peroxidase (HRPO)
- in the glycated human serum albumin (gHSA) purification
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico