A8126
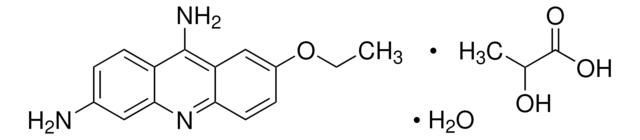
Acriflavine
Powder
Sinónimos:
3,6-Diamino-10-methylacridinium chloride mixt. with 3,6-diaminoacridine (proflavine), Euflavine, Trypaflavine Neutral
About This Item
Productos recomendados
product name
Acriflavine, fluorescent label
form
powder
mp
179-181 °C
solubility
H2O: 0.33 g/mL (lit.)(lit.)
εmax
≥48000 at 459-465 nm in methanol at 0.004 g/L
≥50000 at 259-265 nm in methanol at 0.004 g/L
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
[Cl-].Nc1ccc2cc3ccc(N)cc3nc2c1.C[n+]4c5cc(N)ccc5cc6ccc(N)cc46
InChI
1S/C14H13N3.C13H11N3.ClH/c1-17-13-7-11(15)4-2-9(13)6-10-3-5-12(16)8-14(10)17;14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10;/h2-8H,1H3,(H3,15,16);1-7H,14-15H2;1H
InChI key
PEJLNXHANOHNSU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Excitation and emission wavelengths in various solvents :
- Methanol: λex = 424 nm; λem = 518 nm
- Ethanol: λex = 426 nm; λem = 524 nm
- Propanol: λex = 430 nm; λem = 512 nm
- Butanol: λex = 430 nm; λem = 526 nm
- Formamide: λex = 434 nm; λem = 524 nm
- Glycerol: λex = 432 nm; λem = 540 nm
- Water: λex = 416 nm; λem = 514 nm
Insoluble in ether, chloroform, and fixed oils. Utilized in fluorescence steady state measurements as a donor molecule (when paired with rhodamine 6G as the acceptor) to function as a pH sensor .
- Acriflavine has been used in the agglutination test to distinguish between smooth and rough colony formation of Brucella melitensis.
- It has been used as an Ago2 (argonaute 2) inhibitor.
- It has been used as an inhibitor of HIF-ARNT (hypoxia-inducible factor - aryl hydrocarbon receptor nuclear translocator) complex formation.
- It has been used to study the bacteriocin production by Carnobacterium piscicola.
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico