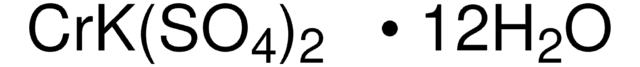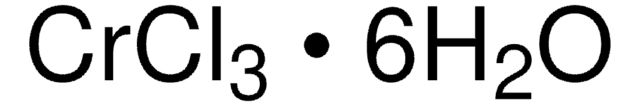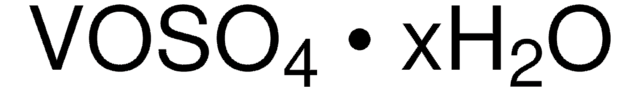243361
Chromium(III) potassium sulfate dodecahydrate
ACS reagent, ≥98%
Sinónimos:
Chrome alum, Potassium chromium(III) sulfate dodecahydrate
About This Item
98.0-102.0% (ACS specification)
Productos recomendados
grade
ACS reagent
assay
≥98%
98.0-102.0% (ACS specification)
form
crystals
reaction suitability
reagent type: catalyst
core: chromium
impurities
≤0.01% insolubles
mp
89 °C (lit.)
anion traces
chloride (Cl-): ≤0.002%
cation traces
Al: ≤0.02%
Fe: ≤0.01%
NH4+: ≤0.01%
heavy metals (as Pb): ≤0.01%
SMILES string
O.O.O.O.O.O.O.O.O.O.O.O.[K+].[Cr+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O
InChI
1S/Cr.K.2H2O4S.12H2O/c;;2*1-5(2,3)4;;;;;;;;;;;;/h;;2*(H2,1,2,3,4);12*1H2/q+3;+1;;;;;;;;;;;;;;/p-4
InChI key
ZFVHBEKVAITXHW-UHFFFAOYSA-J
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
It may be used as a starting material in the synthesis of quasi-monodisperse spherical core-shell particles of Cr/α-Cr2O3 to be used as solar absorbers.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico