16201
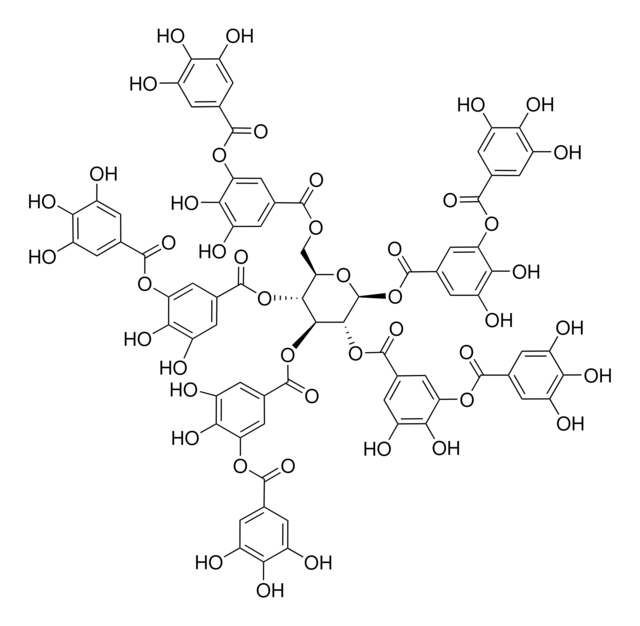
Tannic acid
puriss., powder
Sinónimos:
Gallotannin, Tannin
About This Item
Productos recomendados
grade
puriss.
form
powder
autoignition temp.
980 °F
quality
meets analytical specification of USP
impurities
dextrines, gum matters, in accordance
resinous matters, in accordance
ign. residue
≤0.1% (as SO4)
loss
≤9% loss on drying, 105 °C, 2 h
mp
218 °C (lit.)
solubility
H2O: soluble 1 gm in 0.35ml
glycerol: soluble 1gm in 1 ml (warm)
acetone: very soluble
alcohol: freely soluble (Diluted)
alcohol: slightly soluble (Dehydrated)
alcohol: very soluble
benzene: insoluble
carbon disulfide: insoluble
carbon tetrachloride: insoluble
chloroform: insoluble
diethyl ether: insoluble
hexane: insoluble
petroleum ether: insoluble
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
Oc1cc(cc(O)c1O)C(=O)Oc2cc(cc(O)c2O)C(=O)OC[C@H]3O[C@@H](OC(=O)c4cc(O)c(O)c(OC(=O)c5cc(O)c(O)c(O)c5)c4)[C@H](OC(=O)c6cc(O)c(O)c(OC(=O)c7cc(O)c(O)c(O)c7)c6)[C@@H](OC(=O)c8cc(O)c(O)c(OC(=O)c9cc(O)c(O)c(O)c9)c8)[C@@H]3OC(=O)c%10cc(O)c(O)c(OC(=O)c%11cc(O)c(O)c(O)c%11)c%10
InChI
1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2/t52-,63-,64+,65-,76+/m1/s1
InChI key
LRBQNJMCXXYXIU-PPKXGCFTSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as a reference standard to measure the total hydrolyzable tannins in Oak root bark tannin-methanol extract using a UV/Vis spectrophotometer
- as a blood-brain barrier (BBB) impermeable, transcytosis-inhibiting drug in spheroid culture medium
- as a flushing agent to test its effects on metal ions, total organic carbon, and organic matter of soil
- as an inhibitor of free ovalbumin (OVA) uptake by the endocytic mechanism on dendritic cells
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
390.2 °F
flash_point_c
199 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico