N1090060
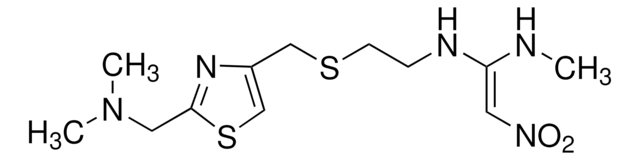
Nizatidine impurity F
European Pharmacopoeia (EP) Reference Standard
Sinónimos:
N1,N1′-[2,4-Thiazolediylbis(methylenethio-2,1-ethanediyl)]bis[N′1-methyl-2-nitro-1,1-ethenediamine]
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C15H25N7O4S3
Peso molecular:
463.60
UNSPSC Code:
41116107
NACRES:
NA.24
Productos recomendados
grade
pharmaceutical primary standard
API family
nizatidine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Nizatidine impurity F EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Related product
Referencia del producto
Descripción
Precios
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico