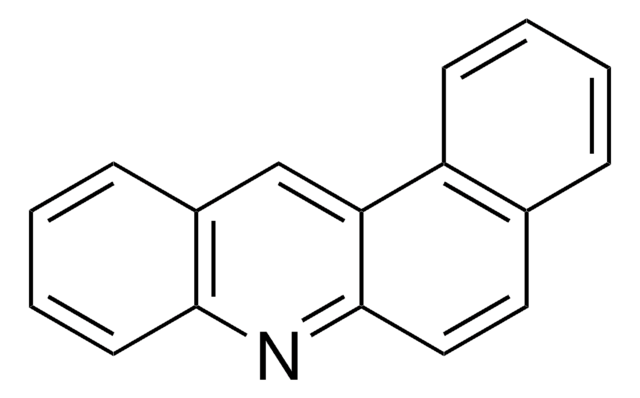
BCR157
Benz[a]acridine
BCR®, certified reference material
About This Item
Productos recomendados
grade
certified reference material
agency
BCR®
manufacturer/tradename
JRC
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
format
neat
storage temp.
2-8°C
SMILES string
c1ccc2nc3ccc4ccccc4c3cc2c1
InChI
1S/C17H11N/c1-3-7-14-12(5-1)9-10-17-15(14)11-13-6-2-4-8-16(13)18-17/h1-11H
InChI key
JEGZRTMZYUDVBF-UHFFFAOYSA-N
Analysis Note
BCR157
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![Dibenz[a,h]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/383/751/70b25872-405f-49b1-b76b-ed5e018ce265/640/70b25872-405f-49b1-b76b-ed5e018ce265.png)
![Benzo[h]quinoline 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)
![4H-benzo[def]carbazole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/319/198/4208123a-456b-4f27-bc62-bc3af7c1d403/640/4208123a-456b-4f27-bc62-bc3af7c1d403.png)
![Dibenz[a,i]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/531/045/5d4c722d-9928-44fb-8bbb-7f4e27442f63/640/5d4c722d-9928-44fb-8bbb-7f4e27442f63.png)
![11H-Benzo[a]carbazole](/deepweb/assets/sigmaaldrich/product/structures/391/065/abfb4cba-81ab-44b8-a816-d8791a903400/640/abfb4cba-81ab-44b8-a816-d8791a903400.png)
![Dibenz[c,h]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/364/643/698df9fb-5b7d-467a-b47e-c8318e2ed298/640/698df9fb-5b7d-467a-b47e-c8318e2ed298.png)