62155
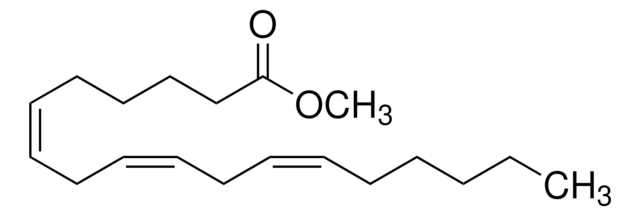
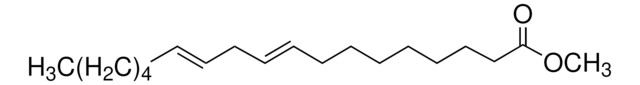
Methyl linolelaidate
analytical standard
Sinónimos:
Linolelaidic acid methyl ester, Methyl trans,trans-9,12-octadecadienoate
About This Item
Productos recomendados
grade
analytical standard
Quality Level
assay
≥99.0% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
format
neat
functional group
ester
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCC\C=C\C\C=C\CCCCCCCC(=O)OC
InChI
1S/C19H34O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h7-8,10-11H,3-6,9,12-18H2,1-2H3/b8-7+,11-10+
InChI key
WTTJVINHCBCLGX-ZDVGBALWSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Packaging
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
gc-analysis-of-a-37-component-fame-mix-g004278
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico