32061
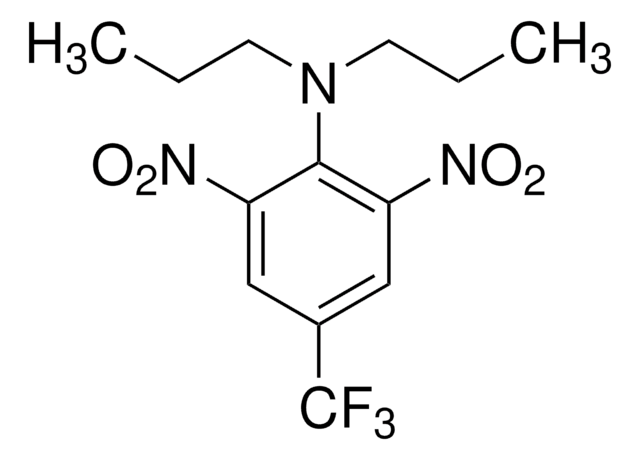
Trifluralin
PESTANAL®, analytical standard
Sinónimos:
2,6-Dinitro-N,N-dipropyl-4-trifluoromethylaniline
About This Item
Productos recomendados
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
CCCN(CCC)c1c(cc(cc1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O
InChI
1S/C13H16F3N3O4/c1-3-5-17(6-4-2)12-10(18(20)21)7-9(13(14,15)16)8-11(12)19(22)23/h7-8H,3-6H2,1-2H3
InChI key
ZSDSQXJSNMTJDA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
ionization (GC-MS-NCI) technique for qualitative and quantitative analysis of trifluralin from soil extract samples.
Legal Information
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
212.0 °F - closed cup
flash_point_c
100.00 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico