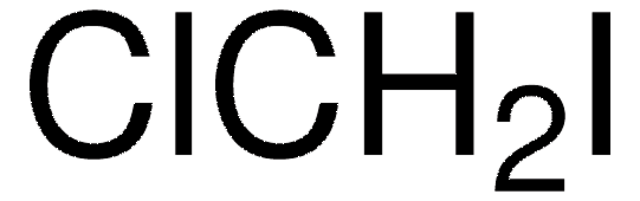
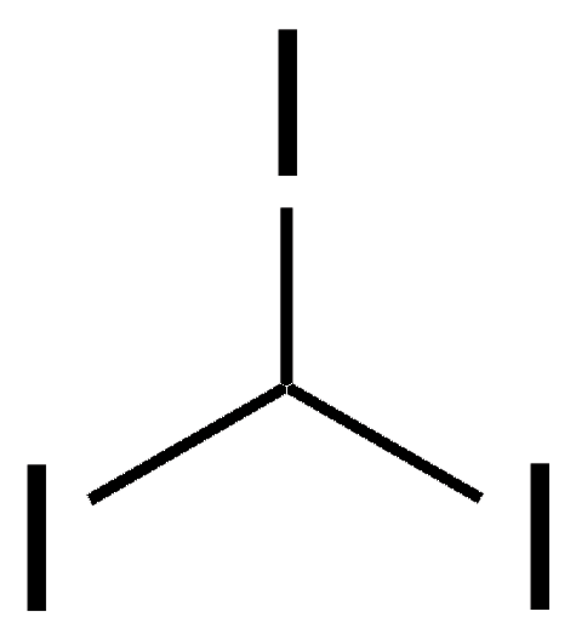
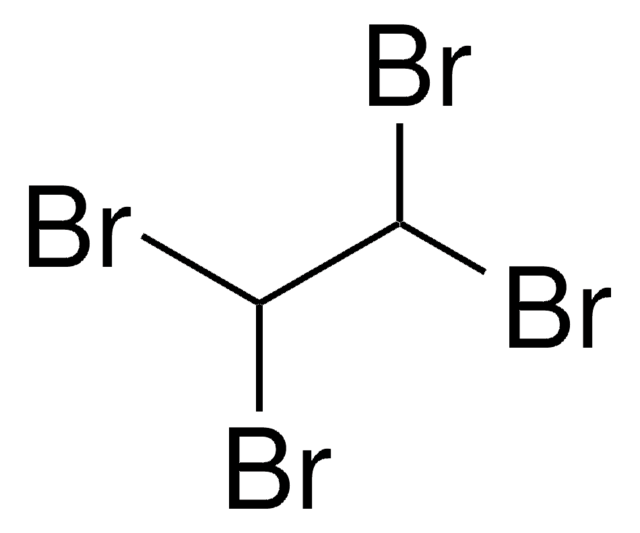
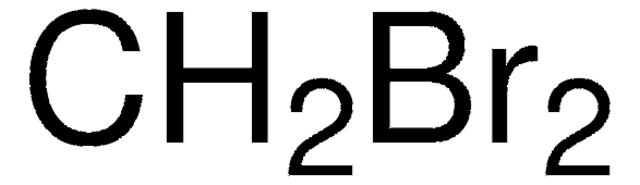158429
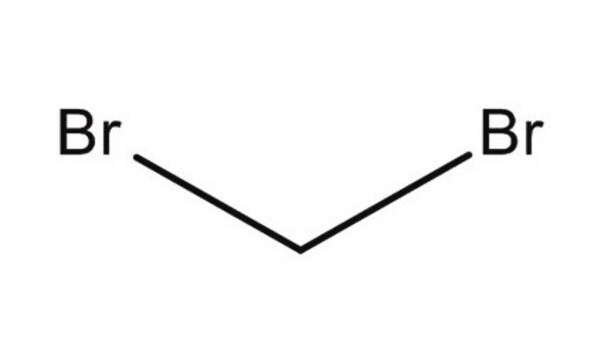
Diiodomethane
ReagentPlus®, 99%, contains copper as stabilizer
Sinónimos:
Methylene iodide
About This Item
Productos recomendados
vapor density
9.25 (vs air)
Quality Level
product line
ReagentPlus®
assay
99%
form
liquid
contains
copper as stabilizer
bp
67-69 °C/11 mmHg (lit.)
mp
5-8 °C (lit.)
density
3.325 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
ICI
InChI
1S/CH2I2/c2-1-3/h1H2
InChI key
NZZFYRREKKOMAT-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Diiodomethane (CH2I2) is an iodine containing organic compound. Its decomposition in acetonitrile initiated by 310nm light has been investigated. Femtosecond pump-probe spectroscopic and ab initio molecular dynamics simulations studies of the photodecomposition of CH2I2 suggest the formation of the isomer of diiodomethane (CH2I-I) as hot photoproduct. In the atmosphere, it undergoes photolysis in the presence of ozone to afford iodine oxide (IO) which results in the formation of aerosols. Its vacuum ultraviolet (VUV) photoabsorption spectrum has been reported.
Application
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico