MAB8121
Anti-Cytomegalovirus Antibody, blend, clone 8B1.2, 1G5.2, and 2D4.2
Chemicon®, from mouse
Sinónimos:
CMV
About This Item
Productos recomendados
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
1G5.2, monoclonal
2D4.2, monoclonal
8B1.2, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
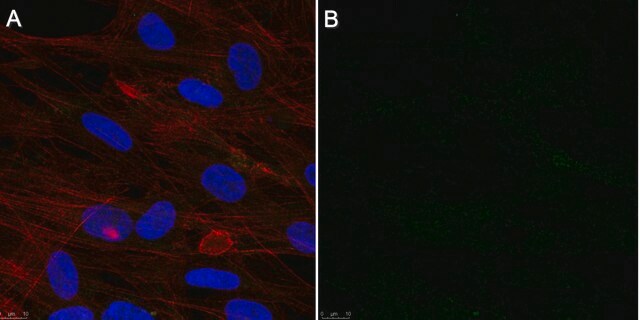
immunofluorescence: suitable
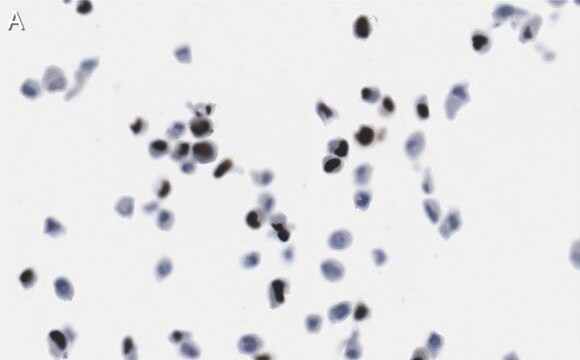
immunohistochemistry: suitable
isotype
IgG2a
shipped in
wet ice
Specificity
With CMV the antigens expressed at different times are listed as:
Immediate Early (alpha gene expression): those antigens expressed at 3-12 hrs post-infection generally involved in Transcription such as 72kD major phosphoprotein and a few other other antigens at 60 - 80kD.
Early Antigen (beta genes) a.k.a. Delayed Early or Intermediate Early: expressed at 12-24hrs post-infection. Generally enzymes and one virion structural gene preceding viral DNA synthesis.
Late Antigen (gamma genes): expressed at 36-48hrs post-infection. Generally structural proteins. Major protein = 55kD
Immunogen
Application
Immunofluorescence
Optimal working dilutions must be determined by the end user.
Physical form
Storage and Stability
Legal Information
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Optional
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico