860076W
Avanti
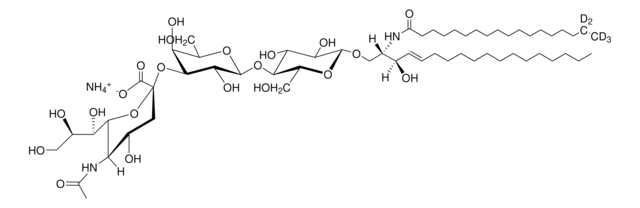
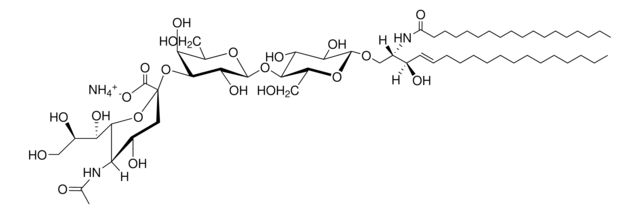
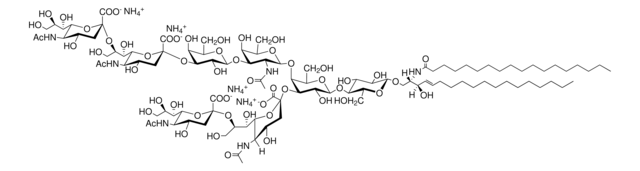
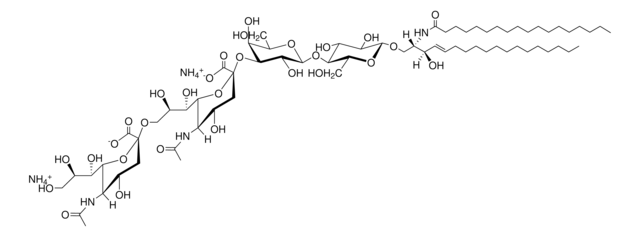
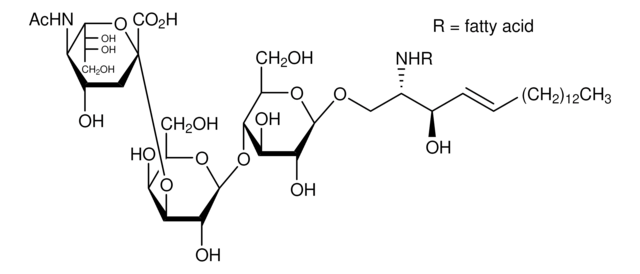
C18:0 GM1-d5
Avanti Research™ - A Croda Brand 860076W, methanol solution
Sinónimos:
C18:0 GM1 Ganglioside-d5 (synthetic, ammonium salt)
About This Item
Productos recomendados
assay
>99% (TLC)
form
methanol solution
packaging
pkg of 1 × 1 mL (860076W-100ug)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860076W
concentration
0.1 mg/mL
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H][C@](/C=C/CCCCCCCCCCCCC)(O)[C@@]([H])(NC(CCCCCCCCCCCCCCCC([2H])(C([2H])([2H])[2H])[2H])=O)CO[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@@H]2O[C@H](CO)[C@@H]([C@H](O[C@@]3(C([O-])=O)O[C@@H]([C@H](O)[C@H](O)CO)[C@@](NC(C)=O)([H])[C@@H](O)C3)[C@H]2O)O[C@@H]4O[C@H](C
General description
Biochem/physiol Actions
Packaging
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F
flash_point_c
9.7 °C
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico