W271705
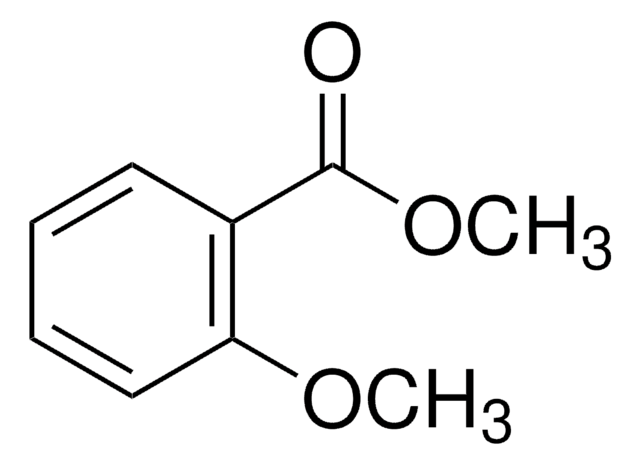
Methyl 2-methoxybenzoate
≥97%
Sinónimos:
Methyl o-anisate
About This Item
Productos recomendados
biological source
synthetic
Quality Level
grade
Kosher
agency
meets purity specifications of JECFA
assay
≥97%
refractive index
n20/D 1.534 (lit.)
bp
248 °C (lit.)
density
1.157 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
hyacinth; herbaceous
SMILES string
COC(=O)c1ccccc1OC
InChI
1S/C9H10O3/c1-11-8-6-4-3-5-7(8)9(10)12-2/h3-6H,1-2H3
InChI key
PFYHAAAQPNMZHO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius.: This study evaluates the repellent effects of Methyl 2-methoxybenzoate among other methyl benzoate analogs against bed bugs, highlighting its potential in developing non-toxic pest control solutions (Strickland et al., 2022).
- Copper-catalysed intramolecular O-arylation: a simple and efficient method for benzoxazole synthesis.: This research uses Methyl 2-methoxybenzoate as a ligand in the synthesis of benzoxazole, a compound with significant pharmaceutical applications, via copper-catalyzed reactions (Wu et al., 2014).
- Attraction of the orange mint moth and false celery leaftier moth (Lepidoptera: Crambidae) to floral chemical lures.: Explores the use of Methyl 2-methoxybenzoate in agricultural pest management, specifically its role in attracting and managing pest populations in crop fields (Landolt et al., 2014).
- Synthesis of (+/-)- and (-)-vibralactone and vibralactone C.: Discusses the synthetic applications of Methyl 2-methoxybenzoate in creating complex organic molecules, such as vibralactone, which are important in medicinal chemistry and biological research (Zhou and Snider, 2008).
Disclaimer
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico