T90301
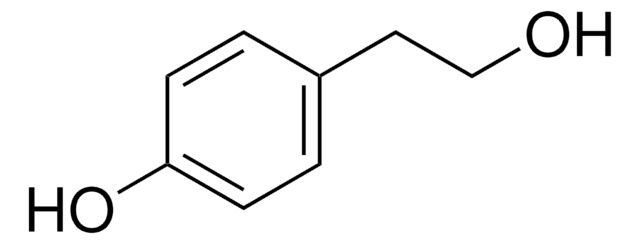
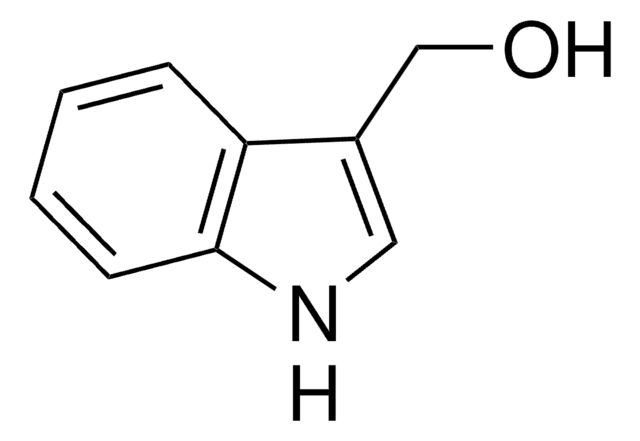
3-(2-Hydroxyethyl)indole
97%
Sinónimos:
2-(3-Indolyl)ethanol, 3-Indoleethanol, IEA, NSC 3884, Tryptophol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H11NO
Número de CAS:
Peso molecular:
161.20
Beilstein/REAXYS Number:
125553
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
mp
56-59 °C (lit.)
SMILES string
OCCc1c[nH]c2ccccc12
InChI
1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
InChI key
MBBOMCVGYCRMEA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Reactant for preparation of:
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Olivier Vandeputte et al.
Applied and environmental microbiology, 71(3), 1169-1177 (2005-03-05)
The role and metabolism of indole-3-acetic acid in gram-negative bacteria is well documented, but little is known about indole-3-acetic acid biosynthesis and regulation in gram-positive bacteria. The phytopathogen Rhodococcus fascians, a gram-positive organism, incites diverse developmental alterations, such as leafy
Richard Pozdrik et al.
Journal of agricultural and food chemistry, 54(17), 6123-6129 (2006-08-17)
The disappearance of riboflavin absorbance at 445 nm from beers or model beers on light exposure is directly linked to light-struck character formation. The addition of (+)-catechin, (-)-epicatechin, tryptophol, or ascorbic acid was able to reduce, but not stop, absorbance
Dina Morshedi et al.
The FEBS journal, 274(24), 6415-6425 (2007-11-22)
Amyloid aggregation of polypeptides is related to a growing number of pathologic states known as amyloid disorders. There is a great deal of interest in developing small molecule inhibitors of the amyloidogenic processes. In the present article, the inhibitory effects
Xuqing Zhang et al.
Organic letters, 7(10), 2043-2046 (2005-05-07)
The tetrahydro-pyrano[3,4-b]indoles 6 were synthesized from 2-(2-trimethylsilanyl-1H-indol-3-yl)-ethanols 5 and various ketones or aldehydes through silicon-directed oxa-Pictet-Spengler cyclizations. An unusual reaction led to the dimeric products 7 when some of 5 was treated with acetone using BF(3) as the catalyst.
Ivan Kosalec et al.
Arhiv za higijenu rada i toksikologiju, 62(1), 41-49 (2011-03-23)
Tryptophol is an aromatic alcohol and secondary metabolite of the opportunistic fungus Candida albicans. Although its toxicity profile at cell level has been poorly investigated, recent data point to cytotoxic, cytostatic, and genotoxic effects in lymphocytes and the induction of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico