H17082
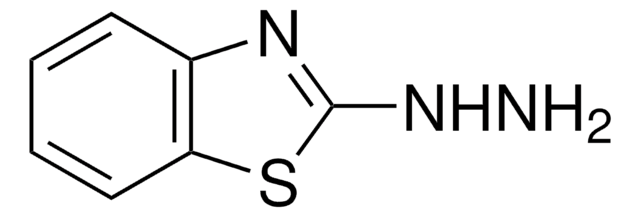
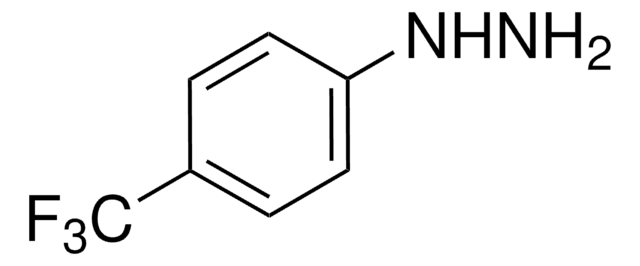
2-Hydrazinopyridine
97%
Sinónimos:
2-Pyridylhydrazine
About This Item
Productos recomendados
Quality Level
assay
97%
bp
90-92 °C/1 mmHg (lit.)
mp
41-44 °C (lit.)
storage temp.
2-8°C
SMILES string
NNc1ccccn1
InChI
1S/C5H7N3/c6-8-5-3-1-2-4-7-5/h1-4H,6H2,(H,7,8)
InChI key
NWELCUKYUCBVKK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Enhanced Metal Removal: Utilizing Schiff base functionalized dialdehyde starch, derived from 2-hydrazinopyridine, demonstrated significant potential in enhancing the removal of Cu(II) from solutions. The study included preparation methodologies, performance evaluations, and DFT calculations, showcasing its efficacy in water treatment technologies (Liang et al., 2024).
- Dual Sensing Probe Development: A novel dicyanisophorone-based probe, incorporating 2-hydrazinopyridine, was developed for the dual sensing of Zn(2+) and Cd(2+) via near-infrared fluorescence. This advancement aids in the detection and analysis of heavy metals in various environmental and biological samples (Yan et al., 2023).
- Active Site Analysis in Lysyl Oxidase: The study provided insights into the spatial arrangement of active site components in Lysyl Oxidase-like 2, including the role of 2-hydrazinopyridine, which is critical for understanding the enzyme′s mechanism and potential therapeutic applications (Meier et al., 2022).
- Structural Analysis of Lysyl Oxidase: Research focused on the predicted 3D structure of the amine oxidase domain of Lysyl Oxidase-Like 2, exploring the interaction dynamics facilitated by 2-hydrazinopyridine. This contributes significantly to the field of molecular biology and enzyme function analysis (Meier et al., 2022).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico