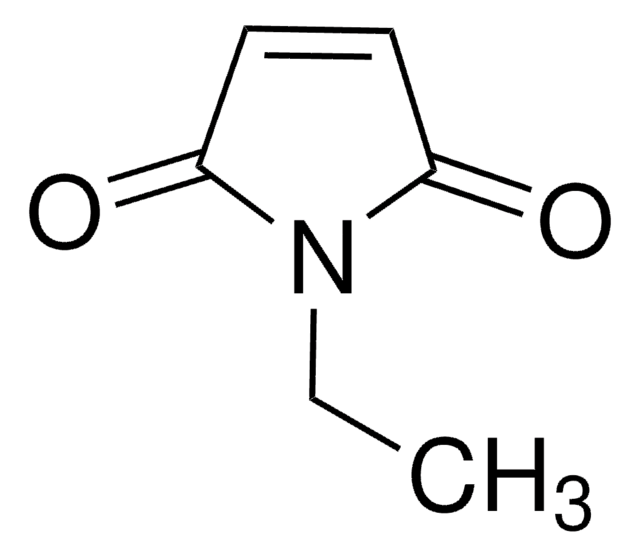
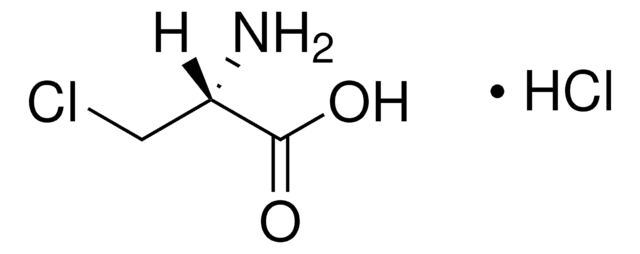
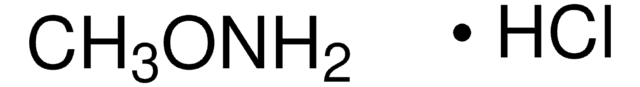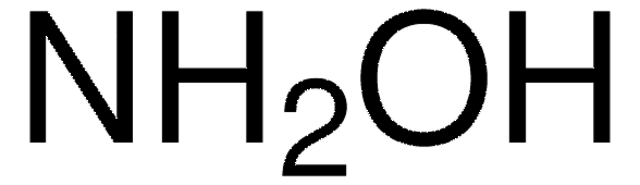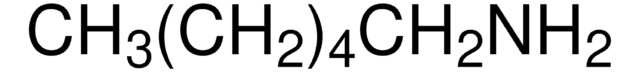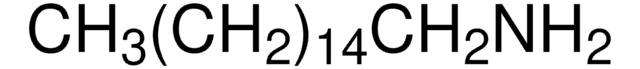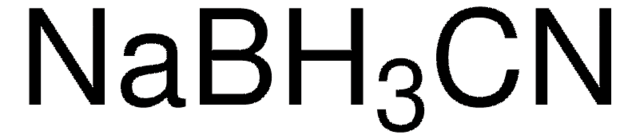C13408
O-(Carboxymethyl)hydroxylamine hemihydrochloride
98%
Sinónimos:
Carboxymethoxylamine hemihydrochloride, (Aminooxy)acetic acid hemihydrochloride, (Carboxymethoxy)amine hemihydrochloride, Hydroxylamine-O-acetic acid hemihydrochloride
About This Item
Productos recomendados
biological source
synthetic (organic)
Quality Level
assay
98%
form
crystalline powder
powder or crystals
mp
156 °C (dec.) (lit.)
solubility
water: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
storage temp.
2-8°C
SMILES string
Cl.NOCC(O)=O.NOCC(O)=O
InChI
1S/2C2H5NO3.ClH/c2*3-6-1-2(4)5;/h2*1,3H2,(H,4,5);1H
InChI key
KBXIJIPYZKPDRU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| C13408-1KG | |
| C13408-250MG | |
| C13408-10G | 4061833467367 |
| C13408-1G | 4061833467374 |
| C13408-25G | 4061832104959 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico