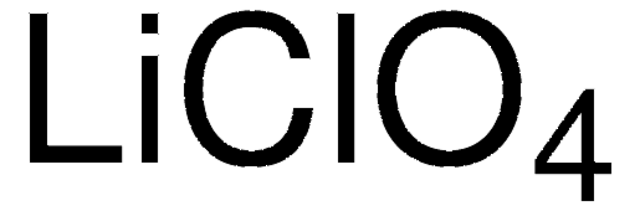931969
Lithium perchlorate
anhydrous, ≥99.9% trace metals basis
Sinónimos:
Perchloric acid lithium salt
About This Item
battery grade
Productos recomendados
grade
anhydrous
battery grade
Quality Level
assay
≥99.9% trace metals basis
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤1000 ppm (trace metals analysis)
pH
6.0-7.5 (25 °C, 5%, aq.sol.)
mp
236 °C (lit.)
solubility
H2O: 59.8 g/dL at 25 °C
anion traces
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
cation traces
Fe: ≤5 ppm
heavy metals: ≤10 ppm
application(s)
battery manufacturing
greener alternative category
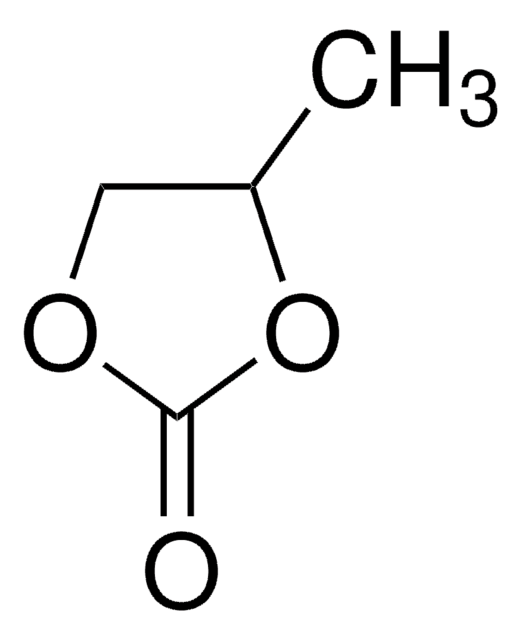
SMILES string
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI key
MHCFAGZWMAWTNR-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Application
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Packaging
500g in poly bottle
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
target_organs
Respiratory system
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico