901058
J51
Sinónimos:
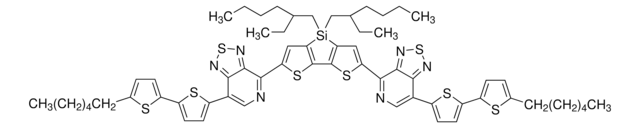
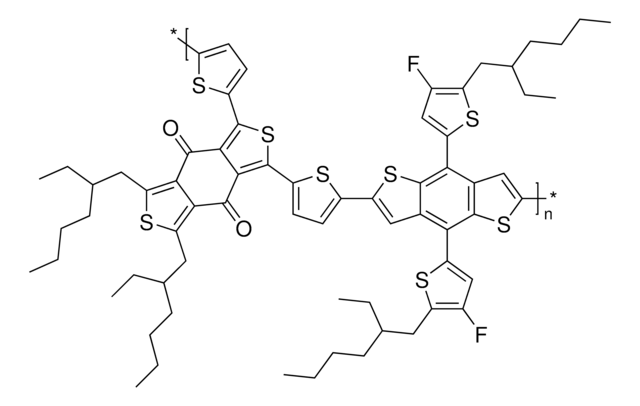
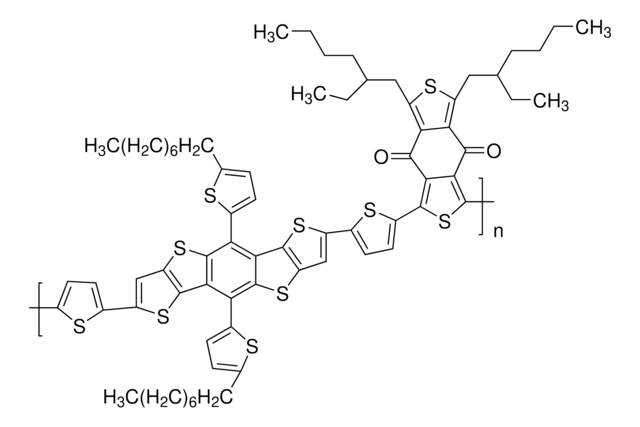
Poly[(5,6-difluoro-2-octyl-2H-benzotriazole-4,7-diyl)-2,5-thiophenediyl[4,8-bis[5-(2-hexyldecyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]-2,5-thiophenediyl]
About This Item
Productos recomendados
description
Band gap: 1.99 eV
Quality Level
form
solid
mol wt
Mw 40,000-80,000 by GPC (PS standard)
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>200 °C
solubility
organic solvents: soluble (Soluble in chlorobenzene and dichlorobenzene, Limited solubility in CHCl3)
orbital energy
HOMO -5.29 eV
LUMO -3.3 eV
PDI
2.0‑3.0
greener alternative category
Categorías relacionadas
General description
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![Poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-(benzo[2,1,3]thiadiazol-4,8-diyl)] average Mn ≤25000](/deepweb/assets/sigmaaldrich/product/structures/428/661/1c4ebb98-9d51-48c0-96c7-e556ca425aa4/640/1c4ebb98-9d51-48c0-96c7-e556ca425aa4.png)