671479
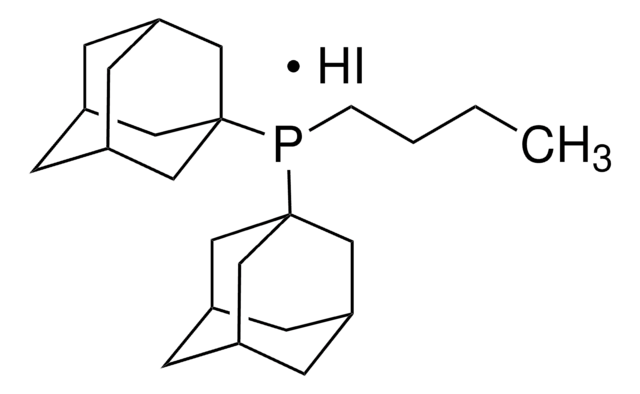
Di(1-adamantyl)-n-butylphosphine
95%
Sinónimos:
cataCXium® A
About This Item
Productos recomendados
Quality Level
assay
95%
reaction suitability
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Cross Couplings
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
functional group
phosphine
SMILES string
CCCCP([C@]12C[C@H]3C[C@H](C[C@H](C3)C1)C2)[C@@]45C[C@@H]6C[C@@H](C[C@@H](C6)C4)C5
InChI
1S/C24H39P/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24/h17-22H,2-16H2,1H3/t17-,18+,19-,20-,21+,22-,23-,24-
InChI key
HTJWUNNIRKDDIV-FECFBMJZSA-N
Categorías relacionadas
General description
cataCXium® A is commonly used as a catalyst in cross-coupling reactions, such as Suzuki and Sonogashira reactions.
Application
Other applications:
- palladium-catalyzed carbonylation of aryl and heteroaryl halides
- palladium-catalyzed synthesis of (hetero)aromatic nitriles
- palladium-catalyzed aminocarbonylation of aryl halides
Features and Benefits
- Mild reaction condition
- Low catalyst loading
- High yield and turn over number
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
cataCXium® - Ligands and Complexes for Efficient Cross-Coupling Reactions. Cross-coupling reactions are an important class of catalytic transformations with applications in polymer science as well as in the fine chemicals and pharmaceutical industries.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico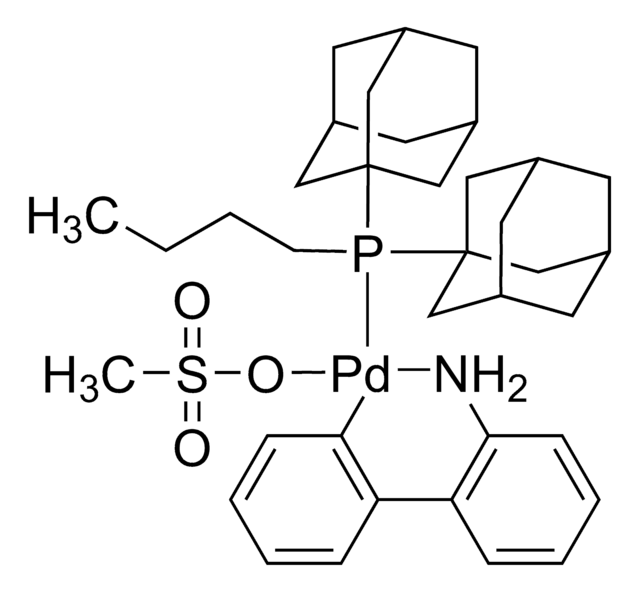

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)

![1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)