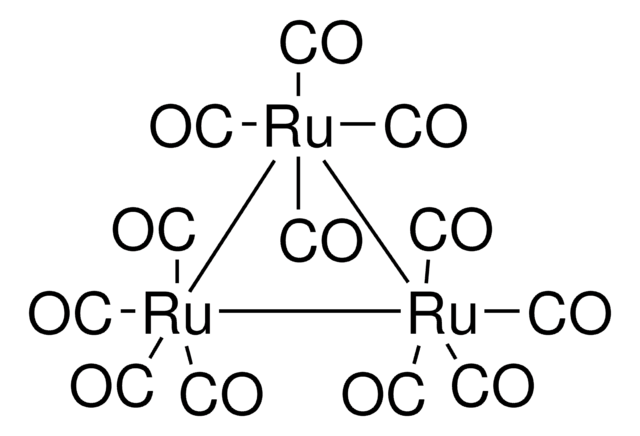
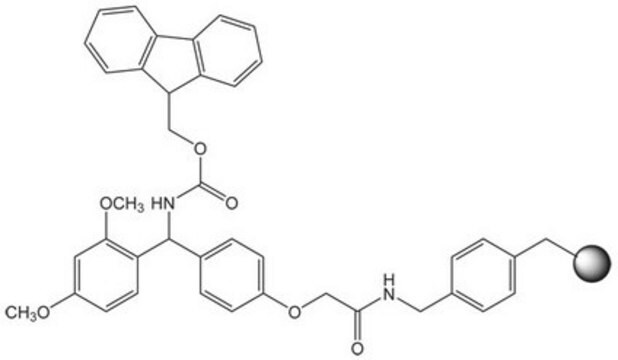
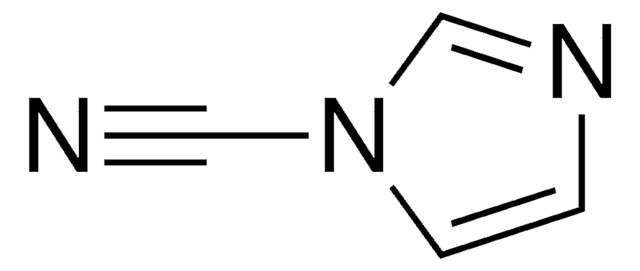
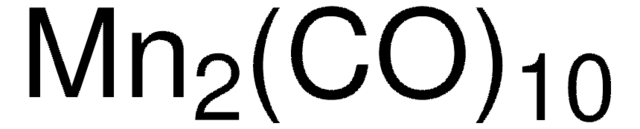60811
Cobalt carbonyl
moistened with hexane (hexane 1-10%), ≥90% (Co)
Sinónimos:
Cobalt tetracarbonyl dimer, Di-μ-carbonylhexacarbonyldicobal, Octacarbonyldicobalt, Dicobalt octacarbonyl
About This Item
Productos recomendados
Quality Level
assay
≥90% (Co)
form
solid
quality
moistened with hexane (hexane 1-10%)
reaction suitability
core: cobalt
reagent type: catalyst
storage temp.
2-8°C
SMILES string
[Co++].[Co++].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+]
InChI
1S/8CO.2Co/c8*1-2;;/q;;;;;;;;2*+2
InChI key
MQIKJSYMMJWAMP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Cobalt carbonyl [Co2(CO)8] is commonly used as a catalyst in the hydroformylation (oxo reaction) of alkenes.
- Along with pyridine, it can be used as a catalyst in the carboxylation of alkenes into corresponding acids and esters.
- It is employed as a key precursor in the preparation of cobalt platinum (CoPt3), cobalt sulfide (Co3S4) and cobalt selenide (CoSe2) nanocrystals.
- It is also used as a reagent in Pauson-Khand cyclizations and Nicholas reaction.
signalword
Danger
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Repr. 2 - Self-heat. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Inhalation
target_organs
Nervous system
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
-9.4 °F
flash_point_c
-23 °C
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico