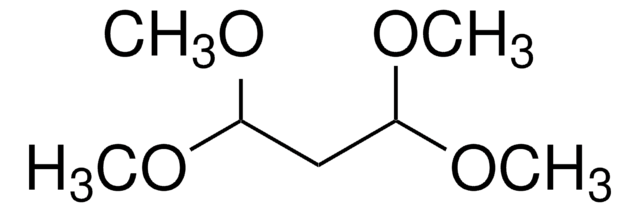
535079
Bromomalonaldehyde
97%
Sinónimos:
Bromomalondialdehyde
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrCH(CHO)2
Número de CAS:
Peso molecular:
150.96
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
mp
132-136 °C (lit.)
functional group
aldehyde
bromo
SMILES string
[H]C(=O)C(Br)C([H])=O
InChI
1S/C3H3BrO2/c4-3(1-5)2-6/h1-3H
InChI key
SURMYNZXHKLDFO-UHFFFAOYSA-N
Categorías relacionadas
Application
Used in the formation of glyoxal-derived adducts from substituted guanines
Used to construct a 1,4-dihydroquinoline bearing a C-3 chiral sulfoxide group which functions as an annelated NADH model in the enantioselective reduction of methyl benzoylformate to methyl mandelate.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Synlett, 441-441 (2005)
E C Taylor et al.
Investigational new drugs, 14(3), 281-285 (1996-01-01)
A new and extremely efficient synthesis of DDATHF from 4-vinylbenzoic acid and bromomalondialdehyde as precursors has been developed which proceeds in 48% overall yield.
Anne-Mari Ruohola et al.
Organic & biomolecular chemistry, 2(13), 1943-1950 (2004-07-01)
Reactions of 9-ethylguanine, 2'-deoxyguanosine and guanosine with bromomalondialdehyde in aqueous buffers over a wide pH-range were studied. The main products were isolated and characterized by (1)H and (13)C NMR and mass spectroscopy. The final products formed under acidic and basic
L Kronberg et al.
Chemical research in toxicology, 6(4), 495-499 (1993-07-01)
Mucochloric acid, a genotoxic compound formed during chlorine disinfection of drinking water, was reacted with adenosine and cytidine at pH 4.0, 90 degrees C. HPLC analyses with UV detection at 325 nm showed that one previously unidentified product peak was
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico