525057
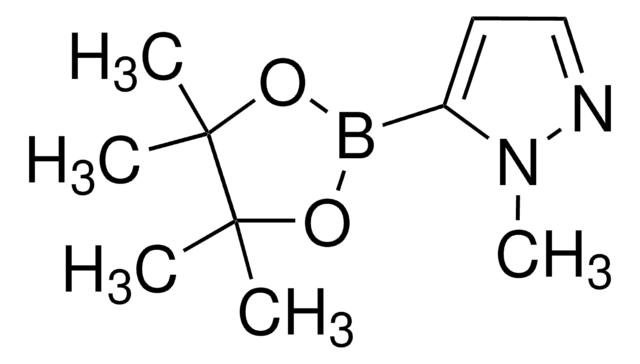
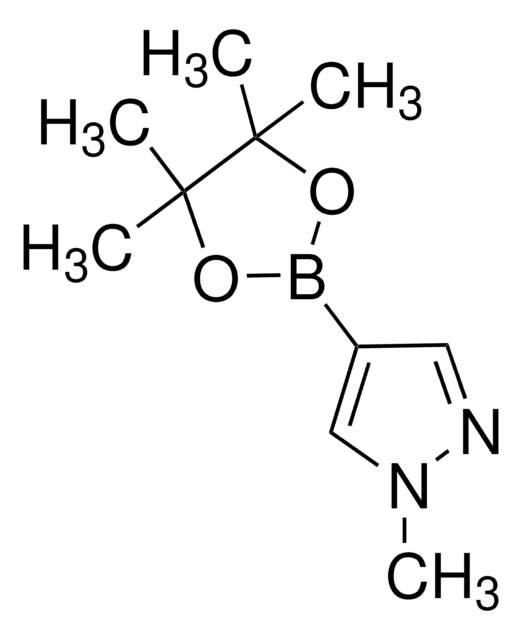
4-Pyrazoleboronic acid pinacol ester
97%
Sinónimos:
4,4,5,5-Tetramethyl-2-(1H-pyrazol-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(1H-pyrazol-4-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(pyrazol-4-yl)-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, Pyrazol-4-ylboronic acid pinacol ester
About This Item
Productos recomendados
assay
97%
form
solid
mp
142-146 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2cn[nH]c2
InChI
1S/C9H15BN2O2/c1-8(2)9(3,4)14-10(13-8)7-5-11-12-6-7/h5-6H,1-4H3,(H,11,12)
InChI key
TVOJIBGZFYMWDT-UHFFFAOYSA-N
Categorías relacionadas
Application
- Suzuki-Miyaura cross-couplings
- Ruthenium-catalyzed asymmetric hydrogenation
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- VEGF
- Aurora
- Rho (ROCK)
- Janus Kinase 2 (JAK)
- c-MET
- ALK
- S-nitrosoglutathione reductase
- CDC7
- Acetyl-CoA carboxylase
- Prosurvival Bcl-2 protein
- Viral RNA-Dependent RNA polymerase
- Long Chain Fatty Acid Elongase 6
- PI3
- AKT
- Chk1
- Protein Kinase B
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)