480894
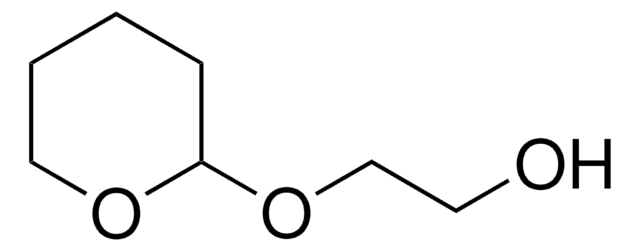
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H11NO2
Número de CAS:
Peso molecular:
117.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
96%
bp
81 °C/20 mmHg (lit.)
mp
34-37 °C (lit.)
functional group
ether
SMILES string
NOC1CCCCO1
InChI
1S/C5H11NO2/c6-8-5-3-1-2-4-7-5/h5H,1-4,6H2
InChI key
NLXXVSKHVGDQAT-UHFFFAOYSA-N
General description
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine (OTX) is an O-substituted hydroxylamine. The coupling of OTX with alkaline gel electrophoresis has been reported to improve the process of detecting single strand breaks (SSBs) in DNA.
Application
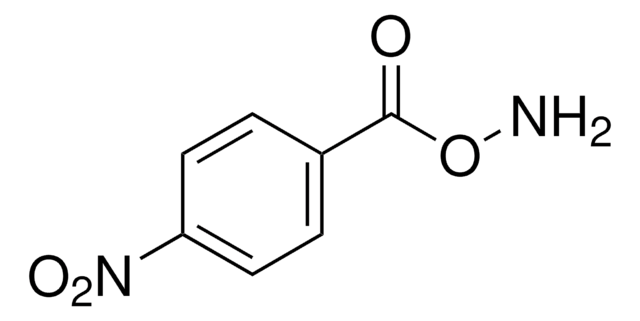
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine may be used in the synthesis of 2-(5-bromothiophene-2-sulfonamido)-N-(tetrahydro-2H-pyran-2-yloxy)acetamides.
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
- 2-[1-(naphthalene-2-sulfonyl)-heterocyclyl]-pyrimidine-5-carboxylic acid (tetrahydropyran-2-yloxy)-amides
- (E)-3-(2-benzyl-1-oxoisoindolin-6-yl)-N-(tetrahydro-2H-pyran-2-yloxy)acrylamide
- 3-(1-benzenesulfonyl-2,3-dihydro-1H-indol-5-yl)-N-hydroxy-acrylamide
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
179.6 °F - closed cup
flash_point_c
82 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chihiro Shinji et al.
Bioorganic & medicinal chemistry, 14(22), 7625-7651 (2006-08-01)
A series of hydroxamic acid derivatives bearing a cyclic amide/imide group as a linker and/or cap structure, prepared during our structural development studies based on thalidomide, showed class-selective potent histone deacetylase (HDAC)-inhibitory activity. Structure-activity relationship studies indicated that the steric
Patrick Angibaud et al.
European journal of medicinal chemistry, 40(6), 597-606 (2005-06-01)
A series of pyrimidyl-5-hydroxamic acids was prepared for evaluation as inhibitors of histone deacetylase (HDAC). Amino-2-pyrimidinyl can be used as a linker to provide HDAC inhibitors of good enzymatic potency.
Elisa Nuti et al.
European journal of medicinal chemistry, 46(7), 2617-2629 (2011-04-26)
Matrix metalloproteinases (MMPs) are important factors in gliomas since these enzymes facilitate invasion into the surrounding brain and participate in neovascularization. In particular, the gelatinases (MMP-2 and MMP-9), and more recently MMP-25, have been shown to be highly expressed in
Tetrahedron Letters, 45, 133-133 (2004)
Han-Li Huang et al.
PloS one, 7(8), e43645-e43645 (2012-08-29)
Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E028, a novel N-hydroxyacrylamide-derived HDAC inhibitor, inhibited human colorectal cancer HCT116 cell growth
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico