459496
9-Borabicyclo[3.3.1]nonane solution
0.4 M in hexanes
Sinónimos:
9-BBN
About This Item
Productos recomendados
reaction suitability
reagent type: reductant
concentration
0.4 M in hexanes
bp
68-70 °C
density
0.691 g/mL at 25 °C
SMILES string
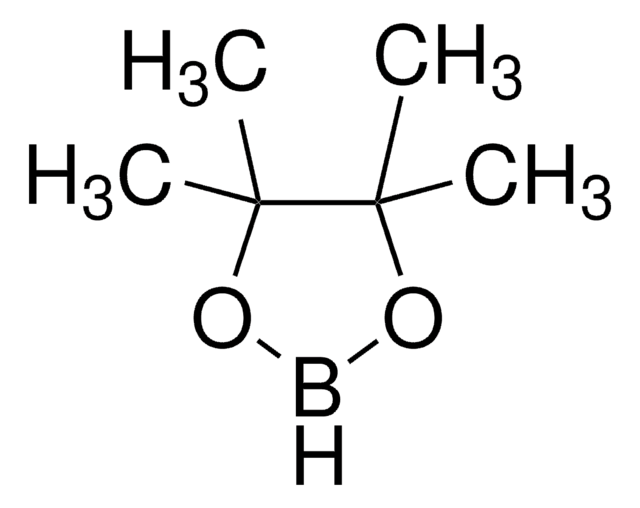
B1C2CCCC1CCC2
InChI
1S/C8H15B/c1-3-7-5-2-6-8(4-1)9-7/h7-9H,1-6H2/t7-,8+
InChI key
FEJUGLKDZJDVFY-OCAPTIKFSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Reactant for:
- Linear SPPS synthesis of ubiquitin derivatives
- Copper-catalyzed cross-coupling reactions of organoboron compounds with primary alkyl halides and pseudohalides
- Intramolecular insertion of alkenes into palladium-nitrogen bonds
- Preparation of (phosphonoacetyl)ornithine to study effect on arginine biosynthetic genes in yeast
- Hetero-Diels-Alder reaction for synthesis of spirocyclic alkaloids
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 Inhalation - STOT SE 3 - Water-react 1
target_organs
Central nervous system, Nervous system
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)
![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)