437875
Cobalt(II) acetate tetrahydrate
99.999% trace metals basis
Sinónimos:
Cobaltous acetate tetrahydrate
About This Item
Productos recomendados
assay
99.999% trace metals basis
form
powder and chunks
reaction suitability
core: cobalt
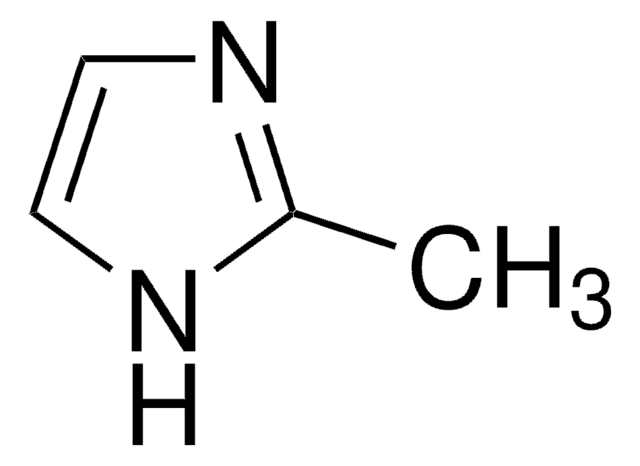
SMILES string
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Co]OC(C)=O
InChI
1S/2C2H4O2.Co.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
InChI key
ZBYYWKJVSFHYJL-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico