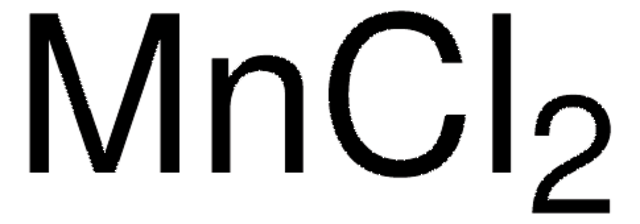429449
Manganese(II) chloride
AnhydroBeads™, −10 mesh, 99.99% trace metals basis
Sinónimos:
Manganese dichloride, Sacchite
About This Item
Productos recomendados
product line
AnhydroBeads™
Quality Level
assay
99.99% trace metals basis
form
beads
impurities
≤150.0 ppm Trace Metal Analysis
particle size
−10 mesh
mp
652 °C (lit.)
density
2.98 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
Cl[Mn]Cl
InChI
1S/2ClH.Mn/h2*1H;/q;;+2/p-2
InChI key
GLFNIEUTAYBVOC-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- A precursor in the preparation of manganese oxide coated natural spherical graphite materials, which are used for improving the electrochemical performance of the graphite materials, making them suitable for applications in batteries and supercapacitors.
- A key component in the preparation of polyvinyl alcohol/manganese chloride composite films, which are find potential applications in areas such as sensors, energy storage devices, and flexible electronics due to their improved optical, mechanical, and thermal properties.
- A precursor in the synthesis of Mn-doped CsPbCl3 perovskite nanocrystals for enhancing their optical properties and quantum yield, which are vital for their application in improving the efficiency of solar cells.
- A catalyst for the transfer dehydrogenation of glycerol to lactic acid.
Legal Information
For use with
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - STOT RE 2
target_organs
Brain
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico